Introduction
Tricuspid atresia is the absence of the tricuspid valve with associated hypoplasia of the right ventricle.
Epidemiology
Tricuspid atresia is the 3rd most common cyanotic congenital heart disease with a prevalence of 1 per 10,000 births. It accounts for 1-3% of all congenital heart anomalies (1-2).
Pathophysiology
The tricuspid valve is absent and the right ventricle is hypoplastic due to absence of the inflow into the right ventricle. In the vast majority of cases there will be a ventricular septal defect (VSD) present and the size of this will affect the size of the right ventricular cavity. In all cases there must be an inter-atrial communication to allow systemic venous return out of the heart via the left atrium and ventricle.
In 70% of cases the great arteries are normally related and so the pulmonary artery arises from the hypoplastic right ventricle. Most commonly in this situation, there is a small VSD present and hypoplasia of the pulmonary arteries. This therefore results in decreased pulmonary blood flow.
In 30% of cases, the great arteries are transposed and so the pulmonary artery arises from the left ventricle and the valve is of normal calibre (2). There can be a tendency for pulmonary over-circulation however the main concern is if the VSD is restrictive, systemic perfusion will be poor.
Coarctation of the aorta or interrupted aortic arch are commonly associated abnormalities and are more common if there is tricuspid atresia with transposed great vessels (2).
Risk factors
No specific risk factors have been identified for tricuspid atresia. Tricuspid atresia is rarely associated with chromosomal abnormalities.
Clinical features
From history
- Poor feeding (if late diagnosis).
Examination
- General: Progressive cyanosis. Aiming for saturations between 75-85%
- Palpation: Systolic thrill associated with pulmonary stenosis is rarely palpable. Hepatomegally may be present if the inter-atrial communication is inadequate or if late diagnosis and child is in heart failure
- Auscultation: Single S2 with pan-systolic murmur due to VSD, best heard at left lower sternal edge. There may also be a continuous, mechanical murmur from the patent ductus arteriosus (PDA)
- Signs of heart failure: very rarely seen unless late presentation or the inter-atrial communication is inadequate (see above)
Investigations
ECG:
- ‘Superior’ QRS axis is seen in most patients with normally related great vessels and in around 50% of those with Transposition of the Great Arteries (TGA). Left ventricular hypertrophy, right atrial hypertrophy and bi-atrial hypertrophy are all common.
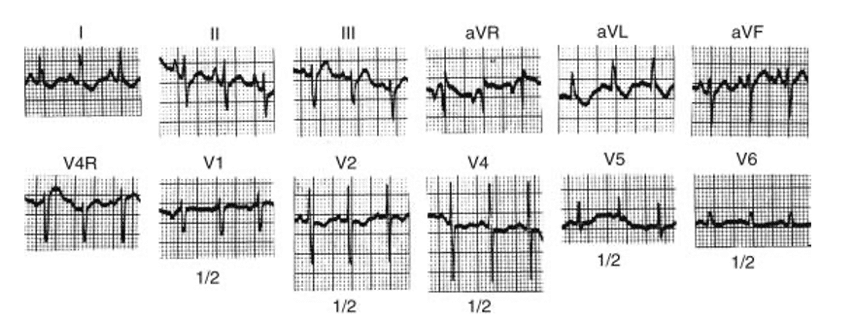
Figure 2: ECG trace of patient with Tricuspid Atresia (4) – note the negative deflection in aVF indicating a superior axis
Chest X-ray:
- Reduced or increased pulmonary vascular markings depending on relation of great vessels
- Heart size may be normal or increased particularly with enlargement of the right atrium and left ventricle
Echocardiogram:
- Atretic tricuspid valve
- Mandatory right to left flow across atrial septum
- Small right ventricle
- Enlarged right atrium, left atrium and left ventricle
- Most important to see the size of the atrial communication and any evidence of restriction of flow as this may indicate the need for a balloon septostomy
- It is also important to look at the size of the VSD, the presence and severity of pulmonary stenosis, relation of the great vessels and for any arch abnormalities
Management
Initial management
-
- IV PGE1 infusion – to prevent closure of PDA
- Balloon atrial septostomy – Rashkind balloon septostomy is required if the inter-atrial communication is inadequate, showing a restrictive flow pattern
Surgical
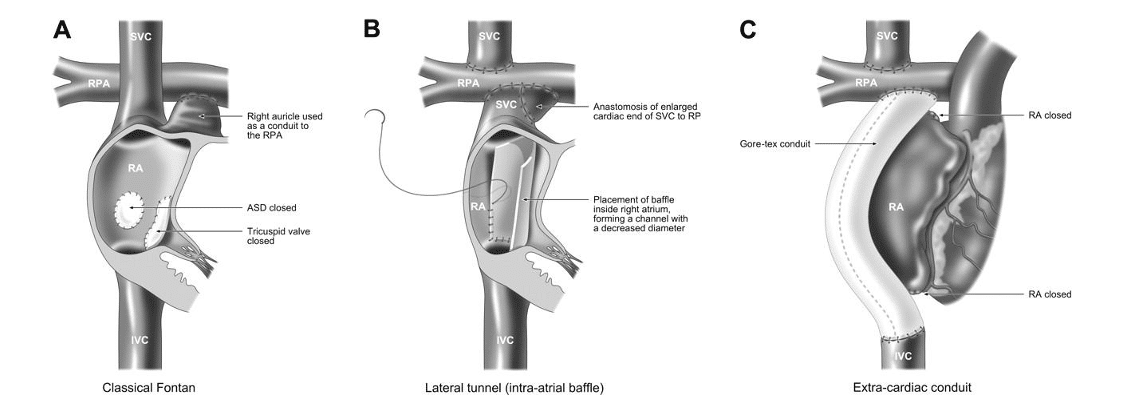
As Tricuspid Atresia causes hypoplasia of the right ventricle, patients embark on the uni-ventricular surgical palliation. The Fontan circulation is the final stage for this pathway and is palliative and not curative. The overall aim of the Fontan circulation is for passive flow of the systemic venous return to go directly to the lungs and therefore avoid the heart. This therefore means that the single ventricle is only required to pump blood to the body, decreasing its workload and so protecting it from earlier failure.
However, for the Fontan circulation to work, you must allow time for the pulmonary vascular resistance to drop. The pulmonary pressures need to be low in order to facilitate forward flow from the low pressure systemic veins. Therefore these patients undergo a series of operations, firstly to augment pulmonary blood flow and allow this time and then to create the Fontan circulation in stages.
Stage 1 (at birth):
Blalock-Taussing (BT) Shunt: Used when pulmonary blood flow is low. This is the most frequently performed first stage operation. A shunt is placed between the subclavian artery and the pulmonary artery to increase forward flow into the pulmonary arteries and so increase pulmonary blood flow.
Damus-Kaye-Stansel (DKS): DKS anastomosis with BT shunt is performed in tricuspid atresia with malposed great arteries and restrictive VSD. This is because there is limited forward flow through the aorta as the VSD is restrictive and the aorta arises off the hypoplastic right ventricle. The DKS procedure involves transection of the main pulmonary artery and distal end sown over. The main pulmonary artery stump is then attached end-to-side to the ascending aorta facilitating forward flow into the aorta. Pulmonary blood flow is then secured with a BT shunt (as above). If there is an associated coarctation of the aorta or interrupted aortic arch, an arch repair will need to be performed at the same time.
PDA Stenting: One option that is now used more are ductal stents placed via a trans-catheter approach to augment pulmonary blood supply. This negates the need for an operation at this stage but cannot be used if there are other abnormalities that need to be surgically addressed and so the above surgical options would be used.
Stage 2 (at around 3-6 months of age):
Bidirectional Cavo-Pulmonary (CP)/Glenn shunt (BGS): BGS creates an end-to-side anastomosis between the superior vena cava (SVC) and the pulmonary artery (PA). The BT shunt is removed at the time of the surgery. If there are bilateral SVCs, then both are anastomosed to the pulmonary arteries and this is called a Bilateral Bidirectional Glenn/CP Shunt.
Hemi-Fontan procedure: The right atrial appendage is attached to the lower margin of the main pulmonary artery. An inter-atrial baffle is then placed in the right atrium to direct SVC flow through to the pulmonary artery. The BT shunt is removed and the native pulmonary valve is over-sewn. This however is now used less with preference towards the bidirectional CP shunt.
Stage 3 (definitive procedure at ~3-4 years):
Fontan procedure: This involves diverting the inferior vena cava flow directly to the PA by either completely bypassing the right atrium (extra-cardiac conduit) or by using a baffle within the right atrium (lateral tunnel). A fenestration is often made between this pathway and the right atrium to allow right to left shunting which may increase cardiac output, although this comes at the expense of mild arterial desaturation (1). Some centres routinely will create a fenestration as they feel that it gives more stability in the initial pre-operative period and can always be closed via a trans-catheter approach at a later stage. Other centres will just use fenestrations in high risk patients (high pulmonary vascular resistance or high PA pressures seen on pre-Fontan catheter, PA stenosis, poor left ventricle systolic or diastolic function with left ventricular end-diastolic pressure (LVEDP) >12mmHg or reduced ejection fraction, atrioventricular valve regurgitation).
The most commonly used procedure currently for Fontan completion is the extra-cardiac conduit. This is because it has been seen to have lower operative mortality, lower incidences of early and late arrhythmias and improved haemodynamics. However the conduit is a fixed size and so will not grow with the patient and there are concerns of longer period of pleural drainage post operatively (2).
All patients following the Fontan operation will be started on some form of anti-coagulation. Depending on the centre, this is either with anti-platelet therapy or with warfarin. At follow up saturations will be monitored and if they significantly drop after exercise and a fenestration is present then they could be put forward for trans-catheter fenestration closure.
Complications (post-Fontan)
Early:
- Low cardiac output and heart failure
- Persistent pleural effusion and chylothorax
- Thrombus formation in venous pathways
Late:
- Supraventricular arrhythmias (also an early complication)
- Protein losing enteropathy, a result of persistent pleural effusion which carries a poor prognosis
- Progressive drop in arterial saturations resulting from obstruction in venous pathways
References
| (1) | Merck Manual: http://www.merckmanuals.com/professional/pediatrics/congenital-cardiovascular-anomalies/tricuspid-atresia |
| (2) | The Pediatric Cardiology Handbook (Park, MK) 5th edition |
| (3) | Herma Heart Institute: https://www.chw.org/medical-care/herma-heart/conditions/tricuspid-atresia |
| (4) | Pediatric Cardiology for Practitioners (Park, MK et al) 4th edition |
| (5) | The Fontan Procedure: Contemporary techniques have improved Long-term outcomes (d’Udekem Y et al) Circulation 2007; 116: 157-164 |