Introduction
Acute leukaemia is the commonest malignancy of childhood. Eighty per cent of children with acute leukaemia have acute lymphoblastic leukaemia (ALL) with the majority of the remainder having acute myeloid leukaemia (AML).1
Epidemiology
AML is diagnosed in approximately 100 children in the UK each year. It can occur at any age with boys and girls being equally affected. It is associated with some genetic disorders and rare conditions and siblings of affected individuals also have a slightly higher increased risk.3
Pathophysiology
AML is a cancer of the blood and bone marrow. Normally, the bone marrow makes blood stem cells that become mature blood cells over time. A blood stem cell may become a lymphoid stem cell or a myeloid stem cell.
A lymphoid stem cell becomes a white blood cell while a myeloid stem cell can become one of three types of mature blood cells:
Acute leukaemia arises from genetic mutations in these blood progenitor cells. These mutations generate both an uncontrollable capacity for self-renewal and the developmental arrest of the progenitor cells at a particular point in their differentiation creating immature cells (blasts).2
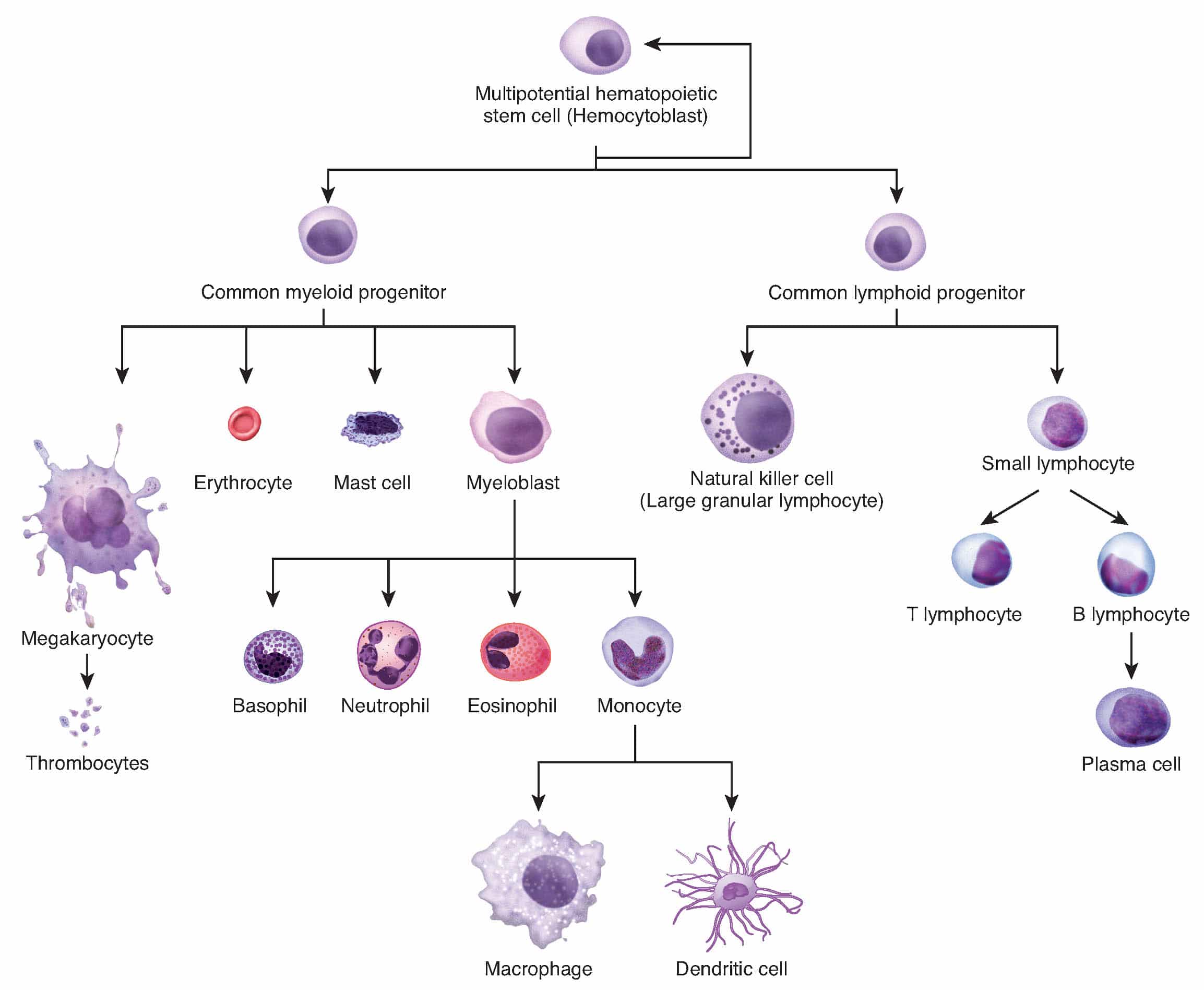
Figure 1: Haematopoiesis
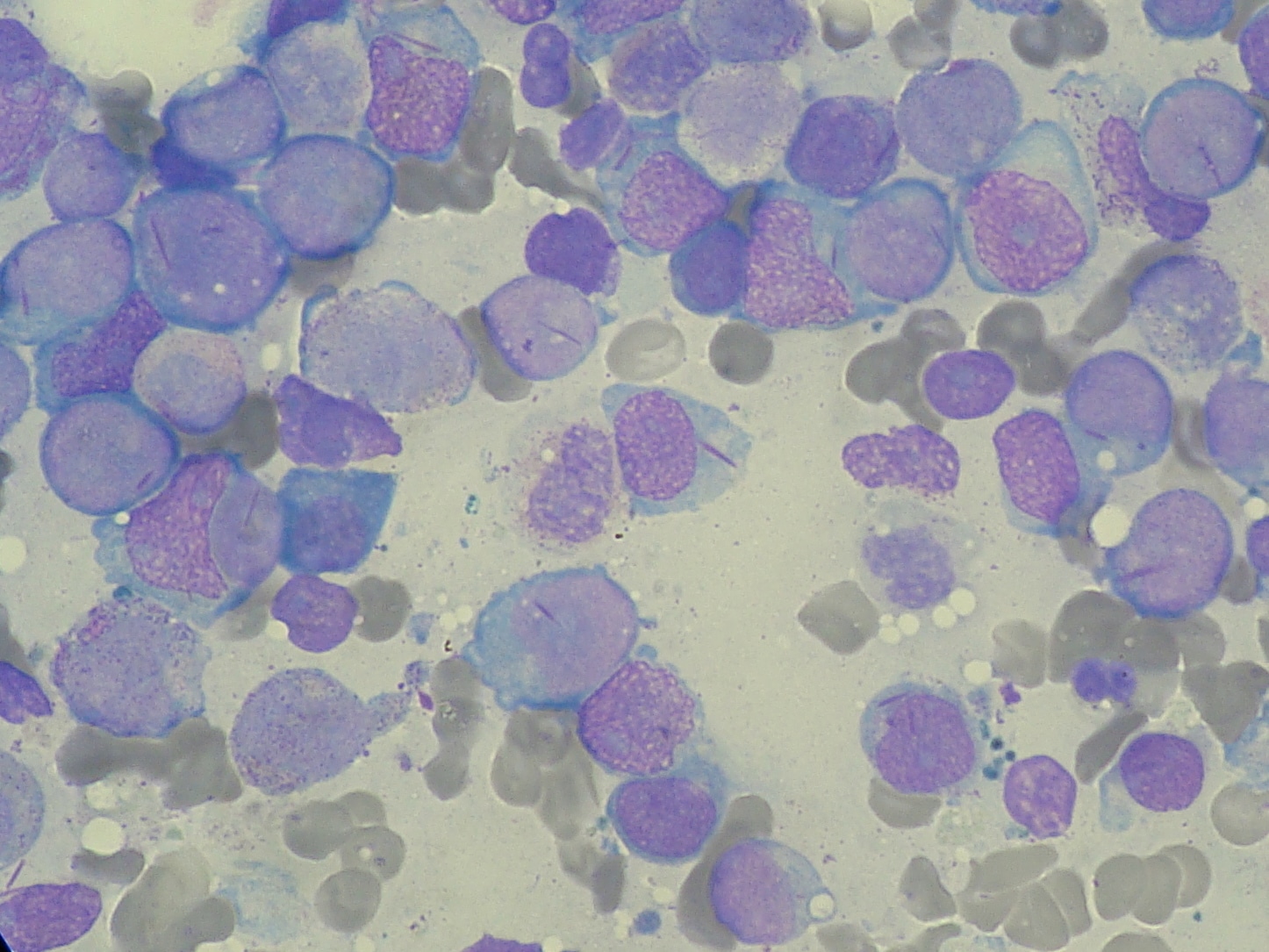
The body is therefore overwhelmed by immature white blood cells (myeloblasts) that infiltrate the bone marrow, reticulo-endothelial system, and other extra-medullary sites. This leaves less room in the blood and bone marrow for healthy cells. When this happens infection, anaemia or easy bleeding may occur.
Childhood AML is diagnosed when the bone marrow has 20% or more blasts. The blasts have the morphologic and histochemical characteristics of one of the French-American-British (FAB) subtypes of AML.4
Table 1: FAB Classification of AML (reproduced from Bennett JM, Catovsky D, Daniel MT, et al). 4
| M0 | AML with minimal evidence of myeloid differentiation |
| M1 | AML without maturation |
| M2 | AML with maturation |
| M3 | Acute promyelocytic leukaemia (APL) |
| M4 | Acute myelomonocytic leukaemia |
| M5 | Acute monocytic/monoblastic leukaemia |
| M6 | Acute erythroleukaemia |
| M7 | Acute megakaryoblastic leukaemia |
A system known as the WHO (World Health Organisation) classification system is also used, which incorporates diagnostic cytogenetic information that can be reliably correlated with outcome.5
The leukaemia cells can spread to the central nervous system, skin, and gums. Occasionally leukaemia cells form a solid tumour called a chloroma or granulocytic sarcoma.
Risk Factors
Some factors, such as genetic disorders and rare conditions, associated with an increased risk of AML include:
- Down’s syndrome
- Li-Fraumeni Syndrome
- Aplastic anaemia
- Myelodysplasia
- Affected sibling (slight increased risk)3
Clinical features
Leukaemia may be strongly suspected when a child presents with classical signs of anaemia, thrombocytopenia, and hepatosplenomegaly or lymphadenopathy. In many cases however, the presenting symptoms are often vague and non-specific, mimicking those of common, self-limiting childhood illnesses.6
Presentations of acute leukaemia relate to three main pathological processes:
- bone marrow failure due to infiltration by blasts
- blast infiltration of other tissues
- systemic effects of cytokines released by leukaemic cells and of increased plasma viscosity (leucostasis) due to extremely high white cell count
Table 2: Features present in AML6-8
| Pathological process | Feature | Symptoms/signs |
| Bone marrow failure | Anaemia | Pallor, lethargy, shortness of breath, dizziness, palpitations, reduced exercise tolerance |
| Neutropenia | Fever, recurrent infections, unusual infections (eg oral candida) | |
| Thrombocytopenia | Bruising, petechiae, epistaxis | |
| Tissue infiltration | Bone marrow | Limb pains |
| Reticuloendothelial | Hepatosplenomegaly, lymphadenopathy, expiratory wheeze (secondary to a mediastinal mass due to lymphadenopathy or thymic infiltration/expansion) | |
| Testes | Testicular enlargement | |
| Systemic effects | Cytokine release | Fever, malaise, fatigue, nausea |
| Leucostasis | Headache, vomiting, cranial nerve palsies, seizures, stroke, shortness of breath, heart failure |
Differential diagnosis
The differential diagnosis of AML depends on the presenting symptom. Differentials include:
- Acute Lymphocytic Leukaemia (ALL) – may present as pancytopenia.
- Iron Deficiency Anaemia – may present as pallor, lethargy and shortness of breath.
- Immune Thrombocytopenic Purpura (ITP) – may present as thrombocytopenia.
- Immunodeficiency – may present as recurrent infections.
Investigations
Laboratory tests
The most important initial investigations are a full blood count and blood film. If both are within normal range, acute leukaemia can be ruled out with a relative degree of certainty. Typically, the full blood count will demonstrate pancytopenia (due to bone marrow infiltration by blasts). Although the patient is likely to be neutropenic, many circulating blasts tend to dramatically elevate the overall white cell count, with blasts clearly visible on blood film.
In some cases, the white cell count may be only slightly raised and, if blasts remain sequestered within the bone marrow, it may even be lower than normal. Clearly identifiable blasts are not always present on a film. In these cases, the only clue that there is a serious underlying problem may be a few atypical cells in the blood film or the presence of leukoerythroblastic features.
Imaging or invasive tests
Some useful investigations to aid diagnosis and management planning include:
- Chest X-ray – information on whether any of the lymph glands in the chest are enlarged.
- Bone marrow aspirate & trephine – bone marrow examination allows definitive diagnosis of acute leukaemia.
- Lumbar puncture – this assesses whether there are any leukaemia cells in the cerebrospinal fluid. Patients may require intra-thecal chemotherapy as part of their treatment.
- Biopsy – AML can also be diagnosed by biopsy of a chloroma.
Information that can be gained from a bone marrow examination includes:
- Aspirate – provides morphological, immunological, and genetic information. This information is used alongside clinical factors (age, sex, white cell count at presentation), and initial response to chemotherapy, to stratify patients according to their risk of subsequent relapse.
- Light microscopy – allows classification as acute lymphoblastic leukaemia (ALL) or acute myeloid leukaemia (AML).
- Immunophenotyping using flow cytometry – identifies patterns of cell surface antigens associated with particular subtypes of acute myeloid leukaemia.
Management
Initial management
The mainstay of treatment is systemically administered chemotherapy. Treatment is usually divided into two phases:
- Induction – induces remission
- Post-remission consolidation/intensification – reduces risk of relapse
Induction
This consists of two cycles of chemotherapy given four weeks apart. Examining the bone marrow after each cycle will allow monitoring of response to induction. The aim is for there to be no evidence of leukaemia in the bone marrow after induction is completed (remission).
Definitive and long-term management
Post-remission therapy
Further chemotherapy is given to destroy any remaining leukaemia cells and to prevent recurrence. Post-remission therapy may consist of varying numbers of cycles of intensive chemotherapy and/or allogeneic haematopoietic stem cell transplantation (HSCT).
Bone marrow transplant
This is reserved for those children with a suboptimal response to standard chemotherapy or if the leukaemia relapses. Approximately 20% of patients with AML will require a transplant.
Maintenance therapy is not part of most paediatric AML protocols; two randomised clinical trials failed to show a benefit for maintenance therapy when given after modern intensive chemotherapy. 9, 10
Complications
The key complications of treatment for acute leukaemia include:
- Neutropenic Sepsis – by far the most important acute complication, which can rapidly trigger overwhelming multi-organ failure.11
- Bone marrow suppression (myelosuppression) – chemotherapy drugs decrease the production of blood cells by the bone marrow for various periods of time; this results in pancytopenia.
- Nausea & vomiting
- Mucositis
- Hair loss
- Long term agent-specific effects
Table 3: Agent specific side effects of chemotherapy
| Chemotherapeutic agent | Side effect |
| Doxorubicin | Cardiotoxicitiy |
| Vincristine | Peripheral neuropathy |
| Cyclophosphamide | Reduced fertility |
| Cytarabine | Hepatotoxicity |
Prognosis
In the early 1980s, the outcome for acute myeloid leukaemia was poor with relapse-free survival rates at 5 years of 18%. However, a 5-year overall survival of 66%, with an event-free survival of 56% was shown by the most recent trial carried out by the UK Medical Research Council.12 Currently, children with low risk cytogenetic abnormalities are identified at diagnosis, and have better prospects of long-term cure than patients with other abnormalities. Patients with adverse cytogenetic features or those who have a poor response to therapy do poorly even with the use of allogeneic bone marrow transplant, with fewer than 20% being cured.
References
| No. | Reference |
| 1. | Vardiman JW, Harris NL, Brunning RD: The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 100 (7): 2292-302, 2002. |
| 2. | Kaleem Z, White G: Diagnostic criteria for minimally differentiated acute myeloid leukemia (AML-M0). Evaluation and a proposal. Am J Clin Pathol 115 (6): 876-84, 2001. |
| 3. | Ebb DH, Weinstein HJ: Diagnosis and treatment of childhood acute myelogenous leukemia. Pediatr Clin North Am 44 (4): 847-62, 1997. |
| 4. | Bennett JM, Catovsky D, Daniel MT, et al.: Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med 103 (4): 620-5, 1985. |
| 5. | Hasle H, Niemeyer CM, Chessells JM, et al.: A pediatric approach to the WHO classification of myelodysplastic and myeloproliferative diseases. Leukemia 17 (2): 277-82, 2003. |
| 6. | Redalli A, Laskin BL, Stephens JM, Boteman MF, Pashos CL. A systematic literature review of the clinical and epidemiological burden of acute lymphoblastic leukaemia (ALL). Eur J Cancer Care 2005;14:53-62. |
| 7. | Alvarez Y, Caballin MR, Gaitan S, Perez A, Bastida P, Ortega JJ, et al. Presenting features of 201 children with acute lymphoblastic leukemia: comparison according to presence or absence of ETV6/ RUNX1 rearrangement. Cancer Genet Cytogenet 2007;177:161-3. |
| 8. | Chessells JM. Pitfalls in the diagnosis of childhood leukaemia. Br J Haematology 2001;114:506-11. |
| 9. | Wells RJ, Woods WG, Buckley JD, et al.: Treatment of newly diagnosed children and adolescents with acute myeloid leukemia: a Childrens Cancer Group study. J Clin Oncol 12 (11): 2367-77, 1994. |
| 10. | Perel Y, Auvrignon A, Leblanc T, et al.: Impact of addition of maintenance therapy to intensive induction and consolidation chemotherapy for childhood acute myeloblastic leukemia: results of a prospective randomized trial, LAME 89/91. Leucámie Aiqüe Myéloïde Enfant. J Clin Oncol 20 (12): 2774-82, 2002. |
| 11. | Pizzo P. Management of fever in patients with cancer and treatment induced neutropenia. N Engl J Med 1993;328:1323-32. |
| 12. | Hann IM, Webb DK, Gibson BE, Harrison CJ. MRC trials in childhood acute myeloid leukaemia. Annals Hematol 2004;83:S108-12. |