Introduction
Atrioventricular septal defects (AVSD) also known as Atrioventricular AV canal defects or Endo-cardiac cushion defects covers a spectrum of congenital cardiac malformations that share a defect of atrioventricular septum and abnormalities of the AV valves (mitral and tricuspid valve). These can be broadly classified into:
- Partial AVSD – Further subtype transitional AVSD
- Complete AVSD – Further subtype intermediate AVSD

Figure 1: Atrioventricular Septal Defect demonstrating mixing of oxygenated and deoxygenated blood [1]
Epidemiology
AVSDs account for up to 5% of all congenital heart defects [2]. They have a strong association with Down Syndrome (Trisomy 21). About 40-45% of children with Down syndrome have congenital heart defects and among these approximately 45% have an AVSD [3].
Familial occurrence of AVSD is rare. Gender distribution is approximately equal or may show a slight female predominance [3].
Complete AVSDs also occur in patients with Heterotaxy Syndromes (an abnormal arrangement of internal thoracic-abdominal organs across the left-right axis of the body).
Aetiology
In normal development, the primitive AV canal connects the atria to the ventricles. At 4-5 weeks of gestation, the superior and inferior endocardial cushions of the common AV canal fuse and contribute to formation of AV valves (mitral and tricuspid) and the AV septum.
Defects arise due to failure of endocardial cushions to fuse correctly leading to apical displacement of AV valves and causing the inlet portion of ventricular septum to be scooped out.
Complete failure of superior and inferior endocardial cushions to fuse causes an ASD (primum atrial septal defect) and VSD (ventricular septal defect) and a single common atrio ventricular valve.
Incomplete fusion between the superior and inferior endocardial cushions results in partial AV canal defects with a primum ASD, a common valvular annulus with two separate AV valve orifices, and a cleft in anterior mitral leaflet.
Pathophysiology
The pathophysiology of these defects depends on the magnitude of blood flow through the septal defect and the amount of atrioventricular valve regurgitation.
Complete AVSD:
There is increased shunting of blood from left to right side of the heart which occurs at both atrial and ventricular levels. In most cases, the pulmonary vascular resistance decreases normally over the first 6 weeks of life, and the patient develops a large left-to-right shunt through both the atrial and ventricular defects.
This in turn causes excessive pulmonary blood flow leading to heart failure and eventually elevated pulmonary vascular resistance. The atrioventricular valves are usually abnormal and incompetent resulting in regurgitation.
Partial AVSD:
Partial defects have left to right shunting at the level of atrial septal defect (primum ASD). This causes volume overload of both right atrium and right ventricle and pulmonary over circulation, but the pulmonary artery pressures are usually normal to mildly elevated. Therefore, the symptoms may be minimal until adulthood or may present in late childhood.
The cleft in the anterior mitral valve leaflet causes regurgitation from left ventricle to left atrium. However, this can also occur from left ventricle to right atrium through the defect, resulting in LV to RA shunt and right sided volume overload.
In AVSDs, the structural changes lead to an increased distance between the aorta and the apex of the heart. This results in an elongation of left ventricular outflow tract (LVOT) and abnormal position of the aortic valve which is displaced anterosuperior rather than being wedged between right and left AV valves. This anatomic change is visualized on echocardiography as “goose neck deformity”. Although the LVOT appears long and narrow, in most cases there is no obstruction.
Clinical features
From history:
- Tachypnoea
- Tachycardia
- Poor feeding
- Sweating
- Failure to thrive (due to excessive metabolic cardiovascular requirements and poor caloric intake) – which in turn is due to tachypnoea.
The severity of these depends on the degree of pulmonary overload and heart failure. Virtually all patients with complete AVSD have symptoms by 1 year of age [3].
From Examination:
General:
Usually an undernourished child who may show characteristics of Down syndrome including flat nasal bridge, up slanting palpebral fissures, prominent inner epicanthal folds, single palmar crease and fifth finger clinodactyly.
Signs of congestive heart failure: hepatomegaly, gallop rhythm, generalized oedema and crackles may be present.
Inspection:
May reveal pallor or Harrison grooves in slightly older children (horizontal depression along lower border of chest at diaphragm insertion site due to chronic tachypnoea).
Palpation:
- A hyperactive precordium,
- Prominent systolic heave along the left sternal border (as a consequence of the volume and pressure overload on the right ventricle)
- Palpable apical thrill (may result due to regurgitation of the atrioventricular valve).
Auscultation:
Complete AVSD
- An accentuated S1
- Loud pulmonary component of S2 – In complete AVSD, the second heart sound narrowly splits and P2 increases in intensity (due to elevated pulmonary artery pressure)
- Ejection-systolic murmur: best auscultated along Left upper sternal border (pulmonary area) due to increased blood flow through a normal pulmonary valve.
- Mid-diastolic murmur: best auscultated along Left lower sternal border and apex due to the increased flow across the common atrioventricular valve.
- Holosystolic murmur: best auscultated along Left lower sternal border and at cardiac apex if left atrioventricular valve regurgitation is present.
NOTE: In complete AVSD, as the VSD is large and unrestrictive, it is not usually associated with a separate murmur
Partial AVSD:
- Wide and fixed splitting of S2: the character of S2 does not change with inspiration
- Ejection systolic murmur: best auscultated at Left upper sternal border due to turbulent blood flow across the pulmonary valve – may radiate to the lung fields.
- Mid-diastolic murmur: best auscultated at Left lower sternal border. Usually low pitched and represents significant left AV valve regurgitation.
- Holosystolic murmur: may be heard at the apex due to regurgitation through anterior mitral cleft
Differential diagnosis
- Isolated Atrial septal defect
- Isolated Ventricular septal defect
- Patent ductus arteriosus– auscultation reveals a continuous machine-like murmur heard throughout systole and diastole
- Paediatric heart failure
- Sepsis – should always be ruled out in any infant presenting with tachypnoea, tachycardia or feed intolerance.
- Other causes of poor weight gain and failure to thrive in infancy should also be ruled out such as malabsorption, nutritional deficiencies etc.
Investigations
Bloods: Due to strong association with Down syndrome, blood sample for Karyotyping should be sent to rule out Trisomy 21.
ECG: Characteristic findings of complete AVSD on ECG include
- Superior QRS axis with the QRS axis between -40 to -150 degrees is characteristic of the defect [2].
- The underlying rhythm is usually sinus but may show prolonged PR interval– secondary to atrial enlargement and increased intra atrial conduction times.
- P wave morphology may indicate right atrial, left atrial of bi-atrial enlargement
- Right ventricular hypertrophy due to right ventricular volume overload- evident as RSR’ pattern in lead V1 may be evident [3].
Radiography: CXR demonstrates cardiomegaly. The pulmonary artery is prominent, and the pulmonary vascular markings are increased.
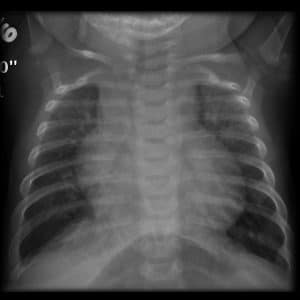
Figure 2: Chest x-ray demonstrating cardiomegaly and pulmonary plethora in a 4m old child with AVSD [4]
Management
Infants with complete AVSD develop heart failure with in the first few months of life. Initial management involves medical therapy but without surgical intervention many of these patients die by the age of 2 to 3 years [2].
Medical Management:
Medical management is focused on symptomatic relief of heart failure. It also buys time for the child to grow and gain optimum weight prior to corrective surgery.
- Diuretics: The main drug from this category is Furosemide, a loop diuretic which relieves pulmonary congestion by reducing the fluid in pulmonary and systemic circulation causing a reduction in preload. Hypo-kalemia is a common side effect of diuretic therapy so Spironolactone (potassium soaring diuretic) is added to minimize the potassium loss.
- Angiotensin Converting Enzyme (ACE) inhibitors: The main drug from this category is Captopril. These reduce the systemic vascular resistance leading to afterload reduction which causes more blood to flow through the left ventricular outflow tract and subsequently reduces the amount of left to right shunt. ACE inhibitors are notorious for increasing serum potassium levels and once these are commenced Spironolactone should be discontinued. Regular monitoring of electrolyte levels is usually required.
- Digoxin: Acts directly on cardiac muscle increasing myocardial systolic contraction. It increases inotropy without increasing myocardial oxygen consumption. It also slows the heart rate and reduces sinoatrial firing.
- Adequate caloric intake: This is essential to ensure adequate weight gain prior to corrective surgery. Often high energy formula feeds are started with daily weight monitoring. In children who are unable to tolerate oral feeding, nasogastric tube feeding should be commenced to ensure caloric requirements are met properly.
The usual approach for management of heart failure in these children is starting with a diuretic and afterload reducing agent while digoxin is added later if further improvement is needed.
Surgical Management:
Complete AVSDs require corrective surgery. Repair must be performed prior to development of irreversible pulmonary vascular obstructive disease and median age of surgery is between 3 to 6 months of age [3].
Children with Down Syndrome have relative pulmonary parenchyma hypoplasia and develop pulmonary vascular obstructive disease earlier. Therefore, this group of children may require surgical correction at an earlier age.
Palliative Surgery:
Palliative surgery involves pulmonary artery banding where a surgical band is placed around the main pulmonary artery in an attempt to decrease the diameter and reduce the pulmonary blood flow and provide relief from the symptoms. With current surgical advances, most centres now perform complete repair in early infancy which has removed the need for pulmonary artery banding. However this strategy is still used in order to allow the child to grow before their full repair for other reasons – e.g. prematurity, low birth weight, deficient mural leaflet and complex complete surgical repair.
Corrective Surgery:
Corrective surgery is carried out via median sternotomy under cardiopulmonary bypass. The aims of surgical repair include:
- Closure of inter atrial communication
- Closure of inter ventricular communication
- Construction of two separate and competent AV valves from available leaflet tissue
Several techniques have been developed to repair AVSD which include:
Single Patch repair:
This is the traditional technique for repair and involves the use of a single patch to close both ASD and the VSD. This patch is positioned by dividing the bridging leaflets. Once the patch is sutured into place, the bridging leaflets are resuspended to the patch.
Double patch repair:
This involves the use of a double patch, a separate patch to close the ASD and the VSD. This is the most commonly used technique. The first patch is utilized to close the VSD deep to the AV valves. The bridging leaflets are not divided. Once the VSD patch is placed and the cleft in left AV valve is repaired, a second patch is utilized for ASD repair.
Modified Single Patch repair:
Also known as the Australian technique, it is one of the more recent techniques and involves the primary suture closure of VSD and pericardial patch closure of ASD resulting in the use of a single patch. Usually used in cases where the VSD is shallow.
A particularly challenging aspect of the repair is dividing the common AV valve found in this condition. Historically some have argued that the cleft of the left AV valve should be considered a true commissure and thus be allowed to persist as a tri leaflet structure. Whereas others have recommended the reconstruction of left AV valve as a bi leaflet structure. Both these techniques have been used by surgeons.
NOTE: If AVSD is complicated by a parachute deformity of the left AV valve, this can result in an obstructed orifice and valve replacement may be required.
Complications
Left untreated, it can lead to significant morbidity and mortality which includes:
- Failure to thrive
- Recurrent lower respiratory tract infections
- Congestive heart failure
- Pulmonary Vascular disease
- Eisenmenger’s syndrome
Following surgical repair complications may include:
- Left AV valve regurgitation – this may persist or worsen due to inadequate surgical reconstruction
- A residual shunt across ASD or VSD which may require a further repair.
- Cardiac conduction defects – Arrhythmias may occur in 10-15% of patients [6]
- Sinus node dysfunction resulting in bradycardia
- Wound infection due to poor healing
Prognosis and outcome
The mortality rate for AVSD is about 2.5% [2]. The mortality rates do not appear to be different in patients with Down syndrome compared with non-syndromic patients when repaired with in the first year of life [7]. Reoperation is required in 15-20% of patients with worsening mitral regurgitation being the leading reason for reoperation [8].
There is no major limitation to exercising and physical activity in children. An Eisenmenger reaction has almost always developed in untreated adolescents and adults with a complete AVSD. The quality of life and physical capacity is significantly impaired in these patients
Postoperatively, lifelong cardiac follow up is needed and should involve monitoring for residual defects, AV valve insufficiency, a subaortic stenosis, development of pulmonary hypertension and arrhythmias.
REFERENCES
| No: | Reference: |
| 1 | https://commons.wikimedia.org/wiki/File:Avsd.jpg |
| 2 | Myung K Park, M.S. (2020). Park’s Paediatric Cardiology for Practitioners. 7th ed. S.L.: Elsevier – Health Science, pp.135–139. |
| 3 | Allen, H.D., Moss, A.J. and Adams, F.H. (2013). Moss and Adams’ Heart disease in infants, children, and adolescents: including the fetus and young adult. 8th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, pp.691–711. |
| 4 | Case courtesy of Assoc Prof Frank Gaillard,
<a href=”https://radiopaedia.org/”>Radiopaedia.org</a>. From the case <a href=”https://radiopaedia.org/cases/7453″>rID: 7453</a> |
| 5 | AVSD_4kammer.jpg (Kjetil Lenes)
https://commons.wikimedia.org/wiki/File:AVSD_4kammer.jpg |
| 6 | Atz, A.M., Hawkins, J.A., Lu, M., Cohen, M.S., Colan, S.D., Jaggers, J., Lacro, R.V., McCrindle, B.W., Margossian, R., Mosca, R.S., Sleeper, L.A. and Minich, L.L. (2011). Surgical management of complete atrioventricular septal defect: Associations with surgical technique, age, and trisomy 21. The Journal of Thoracic and Cardiovascular Surgery, [online] 141(6), pp.1371–1379.
Available at: https://www.jtcvs.org/article/S0022-5223(10)01185-2/fulltext |
| 7 | MASUDA, M., KADO, H., TANOUE, Y., FUKAE, K., ONZUKA, T., SHIOKAWA, Y., SHIROTA, T. and YASUI, H. (2005). Does Down syndrome affect the long-term results of complete atrioventricular septal defect when the defect is repaired during the first year of life? European Journal of Cardio-Thoracic Surgery, [online] 27(3), pp.405–409. Available at: https://academic.oup.com/ejcts/article/27/3/405/448167 |
| 8 | Pontailler, M., Kalfa, D., Garcia, E., Ly, M., Le Bret, E., Roussin, R., Lambert, V., Stos, B., Capderou, A. and Belli, E. (2013). Reoperations for left atrioventricular valve dysfunction after repair of atrioventricular septal defect. European Journal of Cardio-Thoracic Surgery, [online] 45(3), pp.557–563.
Available at: https://academic.oup.com/ejcts/article/45/3/557/438724 |