Introduction
Atrial septal defect (ASD) is the second most common type of congenital heart disease (CHD). ASD occurs when the septum between your left and right atrium is not formed completely. Due to the pressure in left atrium (LA) being greater, ASD is classified as an acyanotic CHD, as oxygenated blood from the LA is being forced through the ASD into the right atrium (RA) (see figure 1).
If the ASD is small enough, it may close without treatment after birth. However, larger defects may require treatment as it can lead to complications.
Epidemiology
ASD is diagnosed in 1.6/1000 live births3. Isolated atrial septal defect accounts for around 5 – 10 % of all CHD and co-exists in around 30 – 50 % of all CHD types1. ASD incidences appear to be on the rise due to the ability of echocardiography in detecting its presence. Females are however more prone to ostium secundum when compared to males, 2:1 ratio.
Pathophysiology
The atrial septum is formed from two separate endocardial cushions, beginning during the 4th week of gestation4. The primary atrial septum is the septum primum, which grows from cranial to caudal; so from the roof of the atrium down towards the atrioventricular endocardial cushions5. This closes off the ostium primum. The ostium secundum develops again from the atrial roof and grows downwards towards the septum primum. The space formed between septum primum and secundum is known as the foramen ovale (FO). FO closes shortly after birth when vascular resistance changes: systemic BP increases with decreasing pulmonary pressure, with a decrease in right atrium pressure6. Figure 1 demonstrates an Atrial Septal Defect.
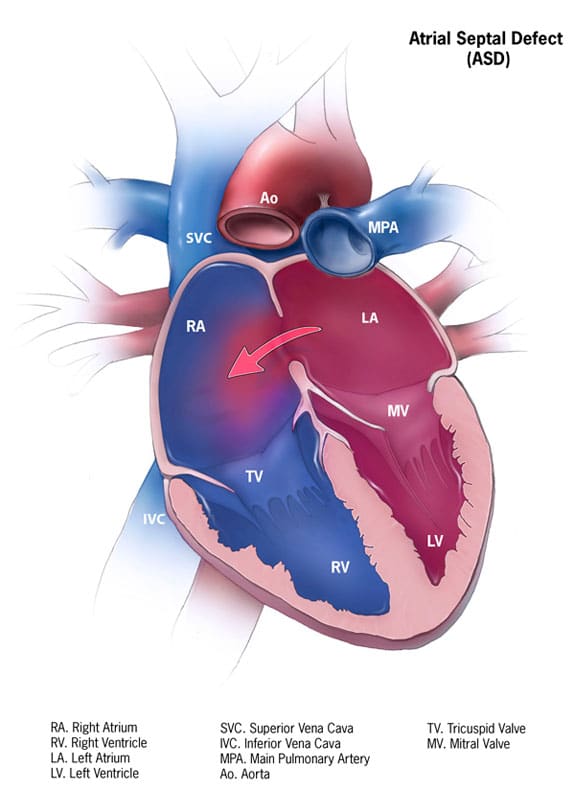
Fig 1. Atrial Septal Defect showing direction of blood flow in normal pressure
There are five types of ASD2, from commonest to least:
- Patent foramen ovale
- Ostium secundum defect
- Ostium primum defect
- Sinus venosus defect
- Coronary sinus defect
Ostium secundum:
Occurs when there is incomplete occlusion of ostium secundum by septum secundum, or too much reabsorption of septum primum from atrium roof (during apoptosis in forming foramen secundum7).
Ostium primum:
Occurs when septum primum fails to fuse with endocardial cushions, allowing blood to travel from left to right atrium. This is the 3rd most common type of ASD5.
There are 2 types of ostium primum defects8:
- Complete AVSD:
A common atrioventricular (AV) valve, but defect spanning from atrium to ventricles. (1 common AV orifice). Check out our atrioventricular septal defect article for more information.
- Partial AVSD:
A partial AVSD is a defect of just the ostium primum, with an intact ventricular septum. (2 separate AV valves and orifices)
Sinus venosus defect:
This can be split into superior and inferior defects:
- Superior defect:
When superior vena cava (SVC) opening runs on top of oval fossa (foramen ovale remnant) of atrial septum. This renders SVC draining blood from both LA and RA. Usually co-exists with abnormal communication between SVC and right superior pulmonary vein5. - Inferior defect:
Less common than superior defect, but occurs when IVC orifice overrides LA & RA. Can co-exist with abnormal communication between IVC and right inferior pulmonary vein5.
Coronary sinus defect9:
Unroofed coronary sinus defect is an absence in the roof of the coronary sinus. This can be partial or focal, allowing transmission between coronary sinus and left atrium.
Risk factors
Autosomal dominance inheritance has been found to have a link with ostium secundum ASD clusters. Risk also increases in children with a family history of ASD10. Certain maternal risk factors identified have been found to be associated with ASDs11:
- Maternal smoking in 1st trimester
- Maternal diabetes
- Maternal rubella
- Maternal drug use e.g. cocaine & alcohol
CHDs are often linked to congenital syndromes, and ASD is no exception. Ostium secundum ASD is commonly associated with syndromes3:
- Treacher-Collins syndrome
- Thrombocytopenia-absent radii syndrome (TAR syndrome)
Although ASD itself is not usually life-threatening, co-existing CHD may increase mortality.
Clinical features
From history
It is important to remember that vast majority of ASD are asymptomatic. Most ASD and PFO are diagnosed following stroke / TIA assessments in adulthood.
Symptoms of large ASD in paediatrics:
- Tachypnoea
- Poor weight gain
- Recurrent chest infections
Symptoms of untreated large ASDs in adults13:
- Exercise intolerance
- Palpitations
- Recurrent chest infections
- Fatigue
- Syncope
From examination
On auscultation14:
- Murmur: soft, systolic ejection murmur, best heard over pulmonary valve region (2nd ICS, figure 2).
- Wide, fixed split S2
- Diastolic rumble in lower left sternal edge in patients with large ASD
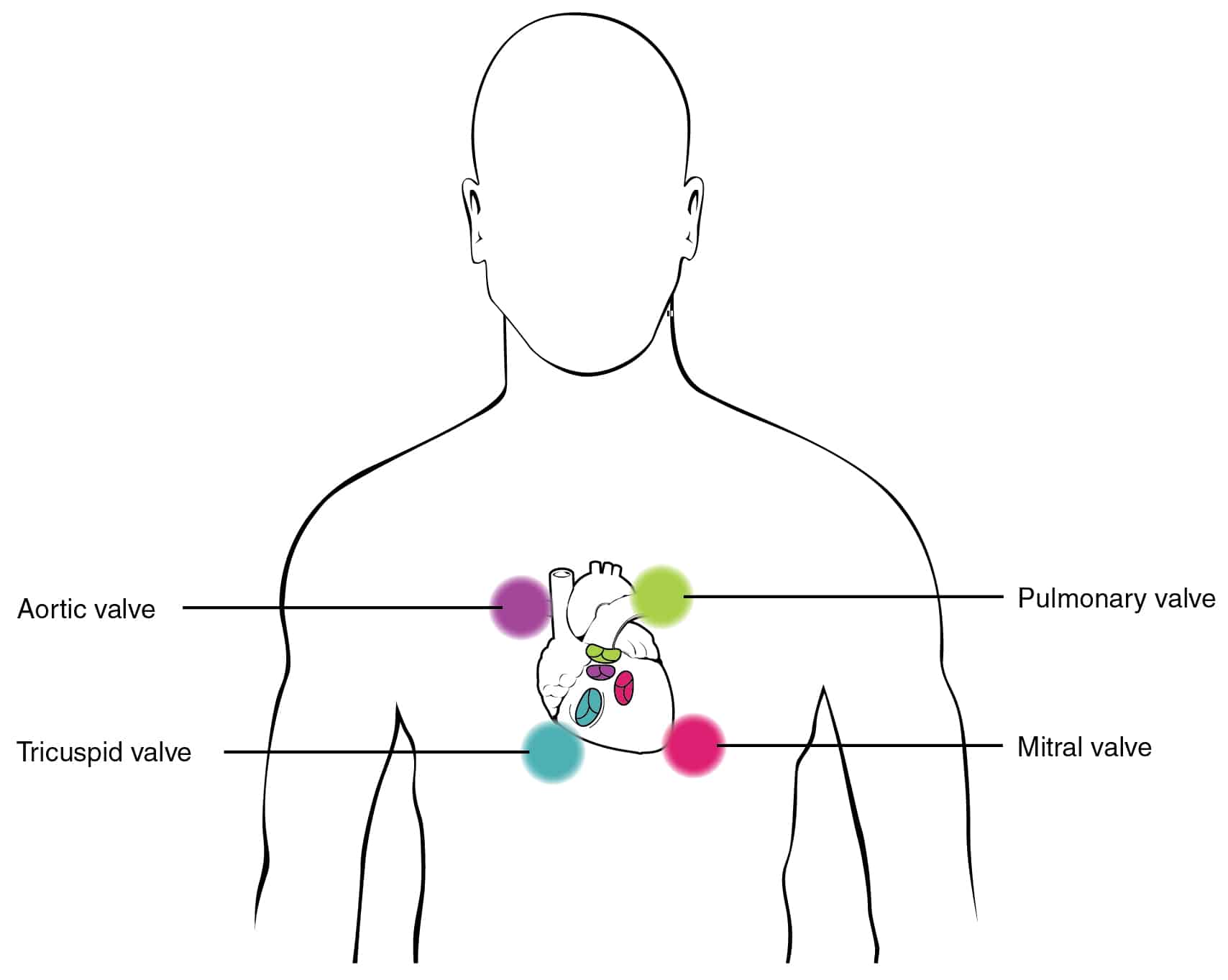
Figure 2: Diagram illustrating the ideal position for listening to specific valves. ASD best heard over upper left sternal edge (pulmonary area)
Differential diagnosis
- Atrioventricular septal defect
- Ventricular septal defect (VSD)
- Innocent murmur: usually soft and musical
- ASD murmurs maximum grade 2/6 with wide, fixed, split S2 (from increasing flow through pulmonary artery)
- Pulmonary stenosis murmur: more turbulent
Investigations
Bedside test
ECG: usually normal in children with small ASD
Possible ECG findings in large ASD:
- Tall P wave (right atrial enlargement)
- Right bundle branch block (incomplete)
- Right axis deviation
In adults, atrial fibrillation (AF) or atrial flutter may be seen.
Imaging or invasive tests3,11
- Transthoracic echocardiogram is gold standard in diagnosing ASD, as it provides information regarding both the size of ASD, and direction of blood flowing through the defect (using Doppler). It is also able to approximate the pulmonary artery pressure.
- Cardiac MRI and CT can also assess the structures around the heart.
- Cardiac MRI, usually carried out in CATH lab measures RV volume, showing RV overload in large ASD.
- CT isn’t imaging modality of choice due to radiation risk
- Cardiac MRI can measure pulmonary vs systemic blood flow ratio (Qp/Qs). The ratio of pulmonary to aortic flow informs us how significant the ASD shunt is.
- CXR identifies cardiomegaly:
- CXR usually normal in children with small shunts
Management
Initial management
Conservative:
If ASD < 5mm, spontaneous closure should occur within 12 months of birth.
In adults, if patient is presenting with no signs of right heart failure and a small defect, then monitor every 2 – 3 years with echocardiogram3.
If presenting with arrhythmia, control rhythm with drugs & anticoagulated before definitive surgical treatment18.
Medical11:
In children with heart failure, diuretics might be needed. Endocarditis prophylaxis is not currently required.
Definitive management19
Surgical closure is the definitive treatment, usually in patients with ASD > 1 cm. Surgery can be carried out percutaneously (transcatheter) or open chest20 (central stenotomy) using cardiopulmonary bypass. Surgical closure is not recommended in patients where pulmonary hypertension is present (mean pulmonary pressure of 30mmHg), as this can induce RV failure if the ASD is closed up.
Percutaneous closure is carried out in cath lab and chosen method dependent on age of child. Percutaneous closure requires adequate septum to be present in order for the devise to hold in place.
Complications of percutaneous closure:
- Arrhythmias
- Atrioventricular block
- Thromboembolism (VTE aspirin)
Indications for surgical closure:
- TIA / stroke
- Ostium primum defects
- Sinus venous defects
- Coronary sinus defects
Complications
Consequences of untreated large ASDs13:
- Arrhythmias (caused by atrial stretch leading to abnormal foci development)
- Pulmonary hypertension
- Eisenmenger syndrome (presenting with: chronic cyanosis, exertional dyspnoea, syncope, increased risk of infections, increased pulmonary vascular resistance)15
- Cyanosis (only if Eisenmenger)
- Peripheral oedema (if eventually leading to heart failure)
- TIA / stroke
Prognosis21
Children born with isolated ASD are expected to have the same life expectancy as general population. If isolated ASD isn’t diagnosed until later life and symptoms develop as a result of ASD, then mortality rate increases. Surgical repair of ASD helps improve symptoms of co-morbidities, but risk of atrial flutter and AF still remain high.
References
| No. | Reference |
| 1. | Hoffman, J. I. E. & Kaplan, S. The incidence of congenital heart disease. Journal of the American College of Cardiology vol. 39 1890–1900 (2002). |
| 2. | Celermajer, D. S. Atrial septal defects: Even simple congenital heart diseases can be complicated. European Heart Journal vol. 39 999–1001 (2018). |
| 3. | Menillo, A. M., Lee, L. & Pearson-Shaver, A. L. Atrial Septal Defect (ASD). StatPearls (StatPearls Publishing, 2020). |
| 4. | Kloesel, B., Dinardo, J. A. & Body, S. C. Cardiac Embryology and Molecular Mechanisms of Congenital Heart Disease: A Primer for Anesthesiologists. Anesthesia and Analgesia vol. 123 551–569 (2016). |
| 5. | Naqvi, N., McCarthy, K. P. & Ho, S. Y. Anatomy of the atrial septum and interatrial communications. Journal of Thoracic Disease vol. 10 S2837–S2847 (2018). |
| 6. | Rudolph, A. M. The Changes in the Circulation After Birth Their Importance in Congenital Heart Disease. http://ahajournals.org. |
| 7. | Van Den Hoff, M. J. B., Van Den Eijnde, S. M., Virágh, S. & Moorman, A. F. M. Programmed cell death in the developing heart. Cardiovascular Research vol. 45 603–620 (2000). |
| 8. | Calabrò, R. & Limongelli, G. Complete atrioventricular canal. Orphanet Journal of Rare Diseases vol. 1 8 (2006). |
| 9. | Bonardi, M., Valentini, A. & Camporotondo, R. Unroofed coronary sinus and persistent left superior vena cava: A case report. J. Ultrasound 15, 179–182 (2012). |
| 10. | Caputo, S. et al. Familial recurrence of congenital heart disease in patients with ostium secundum atrial septal defect. Eur. Heart J. 26, 2179–2184 (2005). |
| 11. | Atrial Septal Defect. Septal defects; information, prognosis | Patient. https://patient.info/doctor/atrial-septal-defect-pro. |
| 12. | Dennis, J., Archer, N., Ellis, J. & Marder, L. Recognising heart disease in children with Down syndrome. Archives of Disease in Childhood: Education and Practice Edition vol. 95 98–104 (2010). |
| 13. | El-Segaier, M. et al. Atrial septal defect: A diagnostic approach. Med. Biol. Eng. Comput. 44, 739–745 (2006). |
| 14. | Naik, R. J. & Shah, N. C. Teenage heart murmurs. Pediatric Clinics of North America vol. 61 1–16 (2014). |
| 15. | Neema, P. Eisenmenger syndrome: An unsolved malady. Annals of Cardiac Anaesthesia vol. 15 257–258 (2012). |
| 16. | Atrial Septal Defect. Septal defects; information, prognosis | Patient. https://patient.info/doctor/atrial-septal-defect-pro. |
| 17. | Mejia, E. & Dhuper, S. Innocent Murmur. (2019). |
| 18. | Warnes, C. A. et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: Executive summary – A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to develop guidelines for the management of adults with congenital heart disease). Circulation vol. 118 2395–2451 (2008). |
| 19. | Yang, M. C. & Wu, J. R. Recent review of transcatheter closure of atrial septal defect. Kaohsiung Journal of Medical Sciences vol. 34 363–369 (2018). |
| 20. | Behjati-Ardakani, M. et al. The clinical course of patients with atrial septal defects. Iran. J. Pediatr. 26, 4649 (2016). |
| 21. | Lindsey, J. B. & Hillis, L. D. Clinical update: atrial septal defect in adults. Lancet 369, 1244–1246 (2007). |
