Introduction
Total anomalous pulmonary venous drainage is a cyanotic congenital heart defect in which the pulmonary veins fail to make their normal connection to left atrium, resulting in the drainage of the pulmonary venous return into the systemic venous circulation.
Epidemiology
- It occurs in about 0.6 to 1.2 per 10,000 live births [1,2]
- It accounts for about 1-1.5% of all congenital cardiac malformations [3]
- There is a marked male preponderance – Male to female ratio – 4:1 (especially for the infra-cardiac type) [4]
Risk Factors
The Baltimore- Washington Infant study (1981-87) has previously shown association with exposure to lead, paint, paint-stripping chemicals or pesticides. There is no known genetic pattern of inheritance associated with this condition to date [5]
Pathophysiology
By definition, no direct communication exists between the pulmonary veins and left atrium. Instead these drain anomalously into the systemic venous tributaries or the right atrium. This results in a mixing of blood between the oxygenated and deoxygenated circuits which result in cyanosis.
To understand this condition better, it is vital to briefly discuss its embryological basis.
Embryology
- The lungs develop as outpouchings from the primitive foregut and therefore share their vascular plexus (splanchnic vascular plexus) at first. This venous drainage is directed into the cardinal and umbilico-vitelline venous systems (primitive SVC and IVC systems).
- At this stage in development, there is no connection between the lungs and the heart.
- At around 4 weeks of gestational age, the primitive pulmonary vein develops as an outpouching from the left atrium.
- This primitive pulmonary vein then connects with the pulmonary venous system that was formed earlier on in development from the splanchnic plexus. The pulmonary portion then separates from the splanchnic plexus.
- In a case of TAPVD, this pulmonary vein either does not form, or, if formed does not connect with pulmonary venous system. This leaves the pulmonary venous system connected to the systemic venous drainage.
- As a consequence the right side of the heart (right atrium and ventricle) remains enlarged at birth (due to all the pulmonary venous return draining to the right side).
- If the pulmonary venous system is neither connected to the primitive pulmonary vein nor the systemic venous system, then it results in either an intra-uterine death or an early neonatal death.
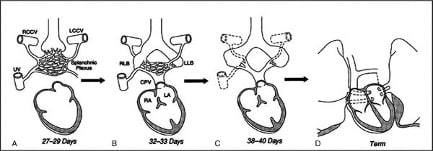
Figure 2: Embryological development of pulmonary veins (RCCV – right common cardinal vein, LCCV – left common cardinal vein, RLB – right lung bud, LLB – left lung bud)
Anatomy
Depending on the site of connections relative to the heart, it is classified into the following types:
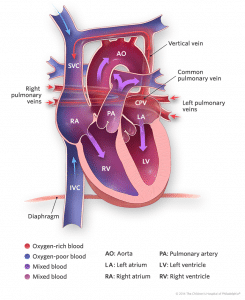
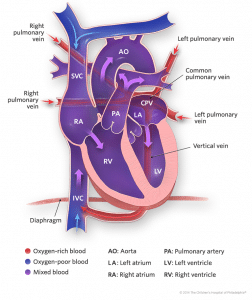
- Supracardiac: It results from retained pulmonary vein connections to the cardinal venous system. The pulmonary veins drain into common confluence behind the left atrium, which then drains into a common vertical vein which then courses to the superior vena cava (right or left) or the azygos vein. This is the most common type of TAPVD and occurs in up to 50% of the cases [6].
- Cardiac: This type of TAPVD also results from retained pulmonary venous connections to the cardinal system. Here the pulmonary veins course toward the heart but instead of draining into the left atrium, they connect to the posterior aspect of the coronary sinus or sometimes the right atrium itself. The coronary sinus normally carries the venous drainage of the cardiac musculature into the right atrium and is usually quite small, but it becomes quite enlarged in this particular condition. It occurs in 15 to 20% of the cases [6].
- Infracardiac: This results from the retained pulmonary vein connections to the umbilico-vitelline venous system. The pulmonary veins drain into a common vertical vein, which then traverses inferiorly through the diaphragm via the oesophageal hiatus and drains into the portal venous system, ductus venosus or less commonly the inferior vena cava. It occurs in about 20% of the cases [6].
- Mixed: This type of TAPVD can be a combination of any one of the above mentioned types. This type occurs in approximately 10% of the cases [6].
Haemodynamics
The three factors that primarily influence the timing of presentation and the severity of symptoms in infants are as follows:
- Pulmonary venous obstruction: This is the most important factor that determines the timing of presentation in these cases.Obstruction can occur at multiple levels:
- Supracardiac level – where the vertical vein courses between left pulmonary artery and left main bronchus.
- Cardiac level – stenosis at the mouth of coronary sinus where the pulmonary veins connect.
- Infracardiac level – constriction of vertical vein at the level of diaphragm or within the liver parenchyma. Infracardiac TAPVD is the type that most commonly presents with obstruction.
Obstruction to the pulmonary venous return results in elevated pressures in the pulmonary venous channels, which is then transmitted to the pulmonary capillary bed. This results in progressive interstitial and alveolar oedema. These changes, in turn, lead to increased pulmonary vascular resistance and elevated pulmonary artery pressure (i.e. pulmonary hypertension). This progressively causes right ventricular dilatation, hypertrophy and ultimately right heart failure. The volume overload of the right ventricle results in a leftward shift of the inter-ventricular septum, leading to a decrease in left ventricular volume. This when compounded with a decreased inflow from left atrium, lead to a decrease in systemic output.
- Inter-atrial communication: The state of the inter-atrial septum is of primary importance in the distribution of mixed venous blood between systemic and pulmonary circulations. In patients with a restrictive inter-atrial communication, the amount of blood reaching the left atrium is limited thereby, compromising the systemic output. Since the pulmonary venous and systemic venous return is to the right atrium, a restrictive inter-atrial communication also results in elevated right atrial pressures, which leads to congestion in both the circuits.
- Left to Right shunting: In unobstructed forms of TAPVD, there is a net left to right shunt due to the gradual decrease in pulmonary vascular resistance after birth. The leads to pulmonary over- circulation over a period of time. This persistently elevated pulmonary blood flow gradually leads to elevated pulmonary artery pressures. If left uncorrected, this eventually leads to right ventricular hypertrophy and right ventricular failure.
Other associations:
- TAPVD most frequently occurs as an isolated anomaly (with normal visceroatrial situs).
- In contrast, patients with visceral heterotaxy of right isomeric type, always have associated TAPVD as the pulmonary veins will have to drain into either one of the morphological right atria.
- It is also occasionally associated with asplenia and cat-eye syndrome
Differential diagnosis
- Respiratory distress syndrome RDS
- Persistent pulmonary hypertension
- Other cyanotic heart conditions : HLHS, TGA, Truncus arteriosus
Clinical features
As explained in the earlier section, the clinical presentations are largely dependent on the presence and degree of pulmonary venous obstruction. Generally, the more severe the obstruction, the earlier and more severe the presentation.
Unobstructed TAPVD
Symptoms:
These infants are relatively stable at birth.
- Asymptomatic at birth
- Mild cyanosis (usually detected on pulse oximetry screening)
- Symptoms due to pulmonary over-circulation: increased work of breathing, recurrent respiratory infections, poor feeding and failure to thrive.
Signs:
- Fixed splitting of second heart sound (due to volume overload of RV)
- Ejection systolic murmur (due to physiological pulmonary stenosis)
- Hepatosplenomegaly (due to right sided heart failure)
- Tachypnea
Obstructed TAPVD
Symptoms:
These infants present severely ill at birth.
- Severe cyanosis
- Respiratory failure
- Shock
Signs:
- Prominent second heart sound
- Soft, continuous murmur heard over area of obstructed anomalous vertical vein
- Weak pulses, low blood pressure and cool peripheries
- Hepatomegaly (due to direct venous congestion)
ECG Features: Usually non-specific findings. They include signs of right ventricular hypertrophy, evident as tall R waves in lead V1 and right atrial enlargement, evident as peaked P waves.
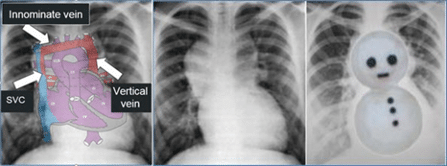
CXR: shows signs of cardiomegaly due to enlarged right atrium and ventricle and pulmonary venous congestion. In older children with a supra-cardiac anomalous pulmonary venous connection, the typical “snowman” or “figure 8” can often be seen, caused by the dilatation of veins (vertical vein and right superior vena cava).
Echocardiography:
ECHO usually helps us to make the definitive diagnosis of this condition. The commonly noted features are:
-
Absent connection of pulmonary veins to the left atrium
-
Abnormal connections of the pulmonary veins to the right sided structures (at the supracardiac, cardiac or infracardiac level).
-
Size and direction of flow across the atrial septal defect
-
Enlargement of right atrium and right ventricle.
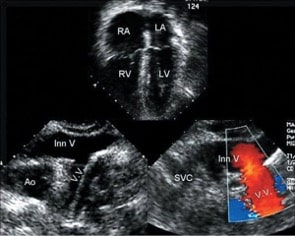
Figure 6: Apical four-chamber view demonstrating right atrial (RA) and right ventricular (RV) dilation. Bottom left: Left-sided vertical vein (VV) connecting to the innominate vein (Inn V). Bottom right: Colour Doppler image demonstrating flow in the vertical vein as it joins the innominate vein. SVC, superior vena cava
Management
Initial Management
The initial management approach is determined by the degree of obstruction and the clinical severity of the case at the time of presentation.
For obstructed TAPVD
This may include routine measures such as:
- Mechanical ventilation – with high peak end expiratory pressures to reduce pulmonary oedema.
- Correction of metabolic acidosis.
- Administration of Prostaglandin E1 – to maintain systemic output.
However the mainstay of management of a case of obstructed TAPVD includes the following measures:
- ECMO (Extracorporeal membrane oxygenation) – can be used as a bridge to definitive repair in these cases. It helps to temporarily bypass the cardiac circulation and to correct severe hypoxemia, acidosis, and to achieve hemodynamic stability.
- Cardiac catheterization – used to perform balloon atrial septostomy in cases with a restrictive atrial communication.
For unobstructed TAPVD
These babies are usually stable at birth. They may develop increased work of breathing due to worsening pulmonary oedema. Symptomatic relief till definitive repair can be achieved by the use of diuretic therapy and other supportive measures.
Definitive repair
Surgical correction is the only definitive treatment and is required in all cases of TAPVD regardless of the degree of obstruction. But the timing of the repair varies with the presence or absence of an obstruction. Any case of obstructed TAPVD is considered a surgical emergency.
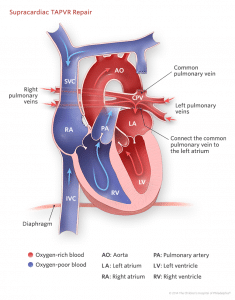
The three main objectives of the surgical repair in all types of TAPVD are as follows:
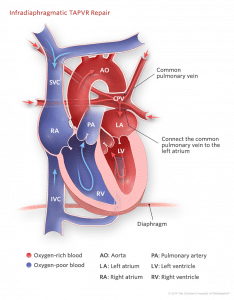
- Connect the common pulmonary venous channel to the left atrium
- Divide the vertical pulmonary vein, and
- Close an residual inter-atrial communication (if present)
The specifics of the procedure, however, vary with the anatomy of the lesion.
- Supra-cardiac type: A side to side anastomosis is made between pulmonary venous sinus and left atrium. The vertical vein is then ligated. The atrial communication is closed with a patch.
- Cardiac TAPVD to coronary sinus: The common wall of left atrium and coronary sinus is excised allowing drainage of pulmonary venous flow into the left atrium. The orifice of the coronary sinus is then closed either by direct suture or with the use of a patch. The atrial communication is closed as well.
- Infra-cardiac type: A vertical anastomosis is made between the pulmonary sinus and the left atrium. The vertical descending vein is ligated above the diaphragm.
Complications
The immediate post-op complications include;
- Pulmonary venous obstruction at the site of surgical repair – requiring long term re-intervention.
- Sinus node dysfunction – due to localised disruption at the site of repair.
- Routine post-op complications: including post-op sepsis and wound infection.
Prognosis
With the advancement of newer treatment modalities including ECMO the survival rate for TAPVD has drastically improved. The early mortality (within 30 days of surgical correction) rates are less than 10% and the late mortality rates are 5%. Long term survival rates to adolescence are between 85-90%. In the absence of residual pulmonary veins stenosis or pulmonary hypertension, exercise tolerance is generally normal in children with repaired TAPVD regardless of the anatomic sub-type.
References
| (1) | Hoffman JI; Kaplan S.The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890 |
| (2) | Reller; Strickland MJ et al: Prevalence of congenital heart defects in metropolitan Atlanta. J Pediatr. 2008; 153(6):807. |
| (3) | Stein, P. (2007), Total Anomalous Pulmonary Venous Connection. AORN Journal, 85: 509-520 |
| (4) | Park’s textbook of pediatric cardiology |
| (5) | Ferencz C;Rubin JD et al. Congenital heart disease: prevalence at livebirth. The Baltimore-Washington Infant Study. Am J Epidemiol. 1985 Jan;121(1):31-6. |
| (6) | Seale AN;Uemura H et al. Total anomalous pulmonary venous connection: morphology and outcome from an international population-based study. Circulation. 2010;122(25):2718. |
Acknowledgments
All the images have been sourced from Children’s Hospital of Philadelphia Cardiac Centre website and Radiopaedia, and being reproduced with their permission.