Introduction
In healthy term neonates, the ductus arteriosus (DA) should close within a few days. When it does not close it is called a patent ductus arteriosus (PDA).
Epidemiology
PDA is associated with preterm neonates (gestational age < 30 weeks) and low birth weight (< 1000 grams)1and accounts for around 5 – 10 % of all congenital heart diseases (CHD)2, occurring in 1 in 2000 term neonates3. PDA is twice as common in females (excluding those associated with congenital rubella infection)2. PDA coexists in around 10% of other congenital heart diseases (CHD)1.
Pathophysiology
The ductus arteriosus is a remnant of the 6th aortic arch. In utero, it connects the pulmonary artery to the descending aorta (right-to-left shunt), allowing foetal circulation to bypass the lungs. Since foetal gas exchange occurs in the placenta, only 10% of blood is circulated through the lungs with 90% passing through the DA2.
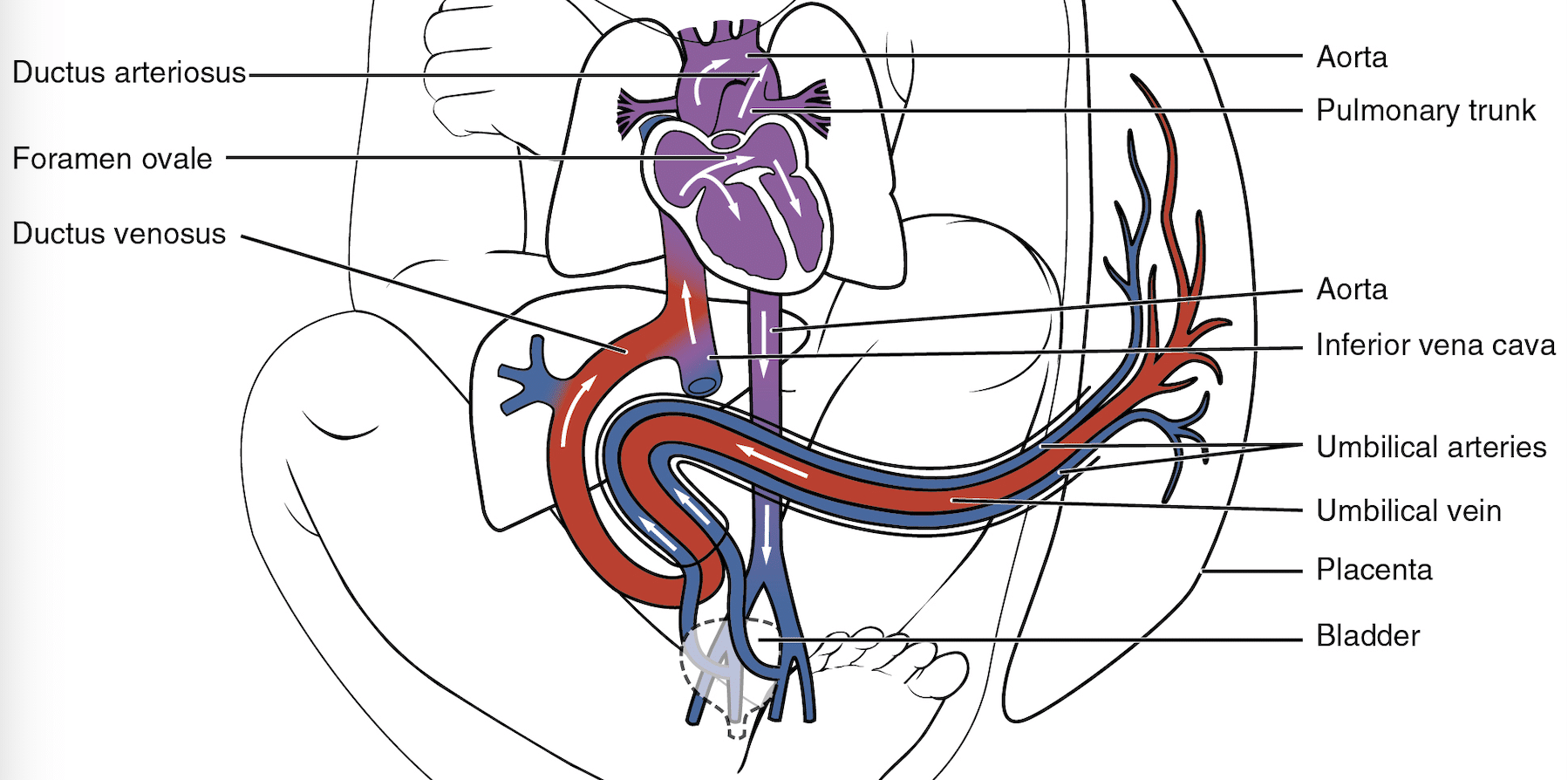
Fig. 1 Diagram showing foetal circulation with direction of blood flow from pulmonary artery through ductus arteriosus to aorta
At birth, chest expansion causes a fall in pulmonary vascular resistance which increases pulmonary blood flow. This causes the ductus arteriosus to close with a few days, eventually forming the ligamentum venosum by 2-3 weeks1. This process is delayed in preterm infants4,5.
In neonates with PDA, the ductus arteriosus remains patent after birth, leaving a connection between the pulmonary artery and aorta. Therefore, oxygenated blood in the aorta flows back into the pulmonary arteries supplying the lungs instead of circulating around the body. The reduced cardiac output to the lower body can cause renal failure and necrotizing enterocolitis1. Tachycardia also occurs secondary to reduced cardiac output.
After birth, PDA becomes a left-to-right shunt (acyanotic), unless the pulmonary pressure exceeds systemic blood pressure. This occurs in persistent pulmonary hypertension of the newborn (PPHN), where there is intermittent reversal of the duct. The ductus arteriosus is classified as PDA post-partum if it persists beyond 3 months in preterm infants or 1 year in term infants6.
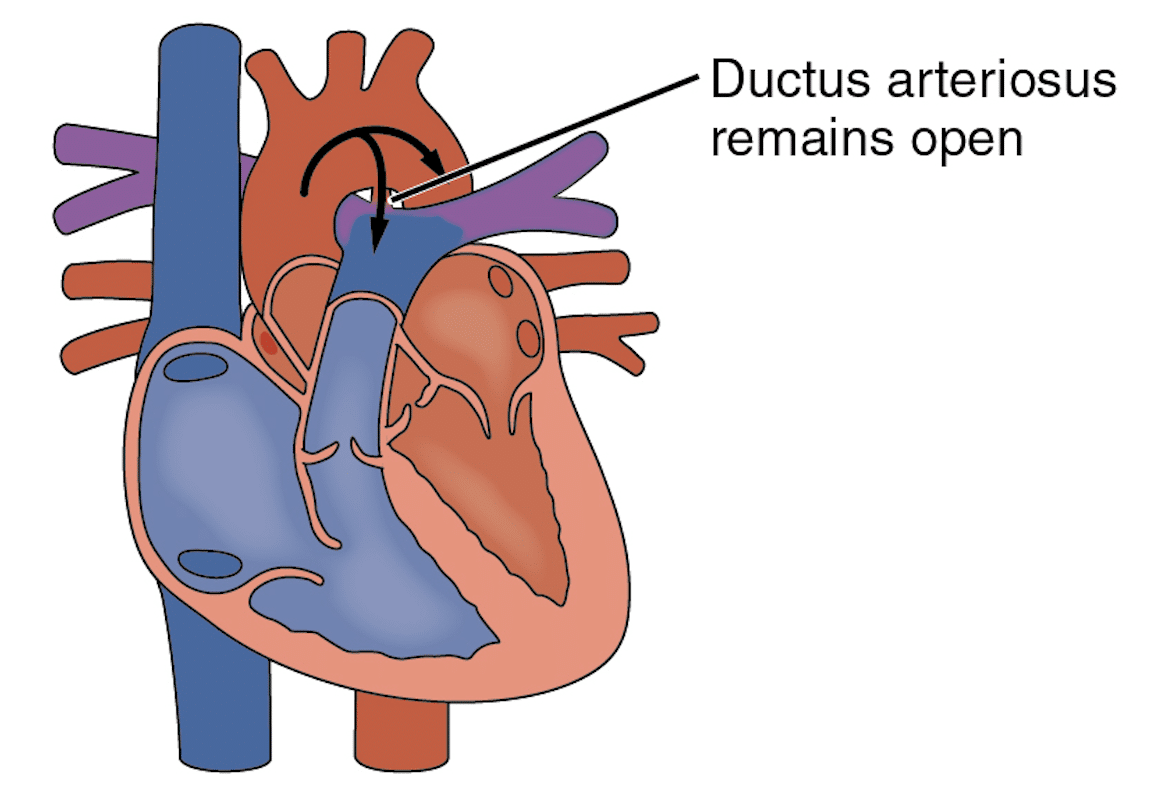
Fig 2. Diagram showing the reversal of blood flow from the descending aorta to the pulmonary artery through the PDA after birth.
Risk factors
The exact mechanism causing PDA is currently unknown, but prematurity has been found to increase the risk of PDA.
Clinical features
From history
Usually asymptomatic if the PDA is small.
If the PDA is large, it can cause2:
- Failure to thrive (secondary to heart failure)
- Feeding difficulties
- Lower respiratory tract infections (LRTI)
Pulmonary oedema and respiratory failure can also occur due to the aorta pumping blood into the pulmonary artery via the PDA causing pulmonary hypertension.
Large PDAs can lead to congestive heart failure due to:
- Excessive blood flow through the pulmonary vasculature from the PDA leads to excessive volumes entering the heart causing left heart dilatation.
- Pumping against increased pressures in the pulmonary vasculature causes right heart failure and hepatomegaly
From examination
A large PDA can result in pulmonary hypertension producing a high left ventricular stroke volume which causes diastolic hypoperfusion3.
This can cause symptoms such as3:
On inspection:
- Tachypnoea
- Respiratory distress
- Episodes of apnoea
- Tachycardia
- Wide pulse pressure (>30mmHg)
On palpation:
- Hyperactive precordium: systolic thrill beneath left clavicle
- Forceful apex beat
- Bounding pulse
On auscultation:
- Continuous crescendo systolic ‘machinery‘ murmur beneath left clavicle (as the shunt is present in both systole and diastole)
- 2nd heart sound concealed by murmur
- Tachycardia
Differential diagnosis1,3
- Large left to right shunt e.g. ventricular septal defect (VSD) and atrioventricular septal defect (AVSD)
- Truncus arteriosus – also produces a continuous murmur
- Venous hum – common benign murmur in children, due to blood draining from brain via internal jugular vein (IJV)
- Aortopulmonary window – an incomplete separation between aorta and pulmonary artery (usually co-exists with other CHDs such as tetralogy of Fallot or transposition of great arteries)11
- Tetralogy of Fallot (with absent pulmonary valve)
- Ruptured aneurysm of sinus of Valsalva (in Marfan’s): abnormal dilatation of aortic root14
Investigations
Bedside tests:
- Bloods: increased serum creatinine due to renal hypoperfusion
- ABG: unexplained metabolic acidosis
- Decreased urine output
Imaging or invasive tests
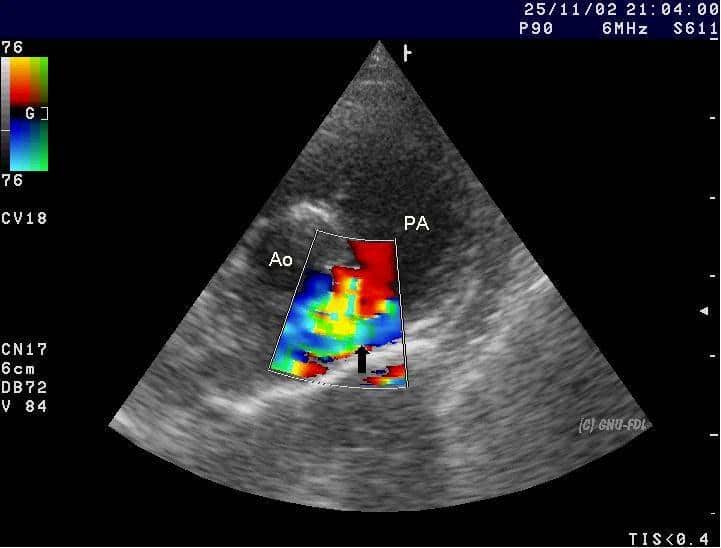
- Echocardiogram:
- PDA is best seen in a short axis view to assess its size in comparison to pulmonary arteries
- Colour flow doppler identifies the direction of blood flow. Usual pattern in PDA is left to right flow (aorta to pulmonary artery). However, it can be from right to left (pulmonary artery to aorta), e.g. in PPHN
- Left heart volume overload with dilated left atrium and left ventricle in a haemodynamically significant PDA
- Chest x-ray:
-
- May show plethoric lung fields due to pulmonary oedema
-
Management1
- Conservative management to await spontaneous closure (usually term infants):
- High calorie formula (failure to thrive): NG feed might be required to reduce child’s work of breathing, especially if preterm
- Careful fluid restriction (110 – 130 ml/kg/d)
- Monitor urine output for renal failure
- Treat pulmonary oedema by increasing peak end-expiratory pressure (PEEP)
- Monitor for necrotising enterocolitis (NEC)
Spontaneous closure with this method usually takes around 7 days in term infants
- Pharmacological management of symptomatic PDA (and preterm infants):
- Indomethacin – may cause necrotizing enterocolitis
- Ibuprofen
- Diuretics
80-90% of infants usually have successful closure of PDA following indomethacin treatment
- Surgical ligation: usually used in infants who are unable to ween from ventilation.
- During surgical ligation, a small metal clip is used to ligate the connection between aorta and pulmonary artery via a left thoracotomy.
Adults usually require surgical ligation, but only if fixed pulmonary hypertension has not yet developed.
- Trans-catheter PDA device closure
- Well established technique for closing PDA in children using coils or duct occluding devices via key-hole technique
- PDA device closure is emerging as a treatment option for pre-term infants with haemodynamically significant PDA and has shown promising results
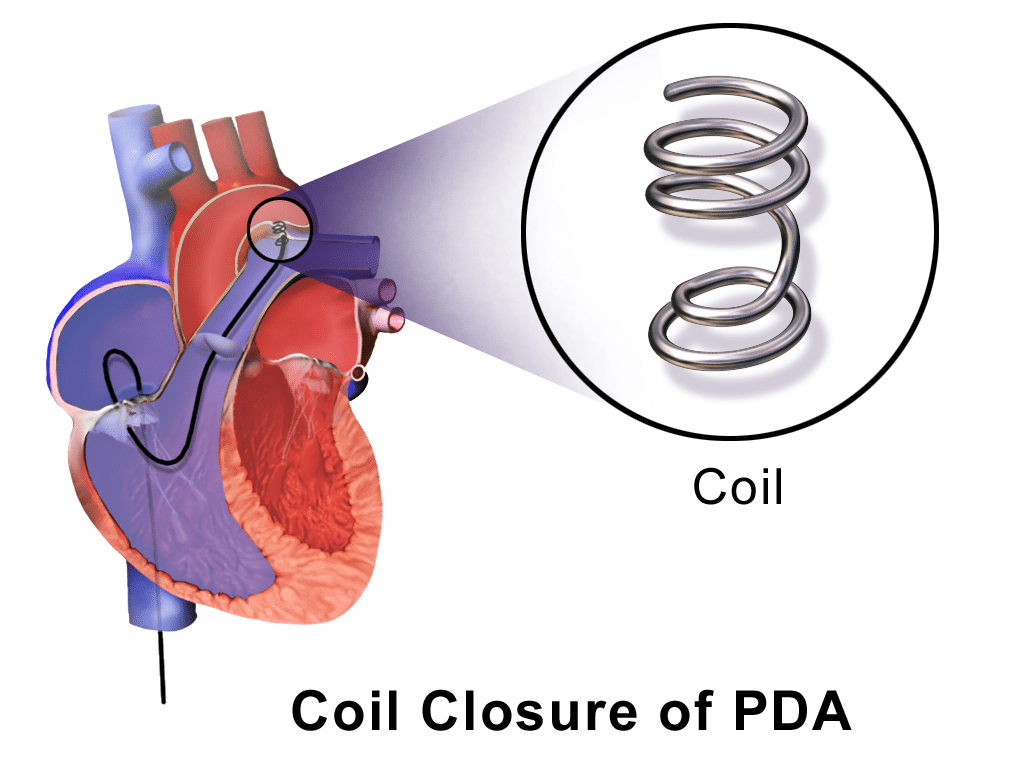
Fig. 4 Diagram showing coil closure of PDA via key-hole technique
Since PDA is a left to right shunt, it increases the risk of endocarditis, necessitating closure in asymptomatic children.
Complications
Untreated patent ductus arteriosus can lead to several complications including:
- Necrotising enterocolitis
- Respiratory distress syndrome (in premature infants)
- Right heart failure
- Pulmonary hypertension (causing premature death)
- Eisenmenger syndrome (very late sign)
Surgical closure of a PDA can lead to complications such as1:
- Injury to descending aorta and surrounding structures e.g. recurrent laryngeal nerve
Closure by trans-catheter device can lead to complications such as:
- Device embolization
- Haemolysis – as a result of residual PDA
Prognosis
The prognosis for PDA is usually good, especially if it is small. Most children are expected to have a normal life expectancy after PDA closure, although spontaneous closure chances are extremely low1.
References
- Ungerleider, G. D., Williams, D. A. & Ungerleider, R. M. Patent ductus arteriosus. in Critical Heart Disease in Infants and Children 536–543 (Elsevier, 2018). doi:10.1016/B978-1-4557-0760-7.00043-7.
- Patent Ductus Arteriosus. PDA information; Treatment | Patient. https://patient.info/doctor/patent-ductus-arteriosus.
- Dice, J. E. & Bhatia, J. Patent ductus arteriosus: an overview. J. Pediatr. Pharmacol. Ther. 12, 138–13846 (2007).
- Fink, D., Nitzan, I., Bin-Nun, A., Mimouni, F. & Hammerman, C. Ductus arteriosus outcome with focus on the initially patent but hemodynamically insignificant ductus in preterm neonates. J. Perinatol. 38, 1526–1531 (2018).
- Jasani, B., Weisz, D. E. & McNamara, P. J. Evidence-based use of acetaminophen for hemodynamically significant ductus arteriosus in preterm infants. Seminars in Perinatology vol. 42 243–252 (2018).
- Anilkumar, M. Patent ductus arteriosus. Cardiology Clinics vol. 31 417–430 (2013).
- Nizarali, Z., Marques, T., Costa, C., Barroso, R. & Cunha, M. Patent Ductus Arteriosus: Perinatal Risk Factors. J Neonatal Biol 1, 3 (2012).
- Ata, Y. et al. Coronary arteriovenous fistulas in the adults: natural history and management strategies. (2009) doi:10.1186/1749-8090-4-62.
- Orphanet: Congenital systemic arteriovenous fistula. https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=en&Expert=2039.
- Ahn, S., Han, J., Kim, H. K. & Kim, T. S. Pulmonary arteriovenous fistula: Clinical and histologic spectrum of four cases. J. Pathol. Transl. Med. 50, 390–393 (2016).
- Law, M. A. & Mahajan, K. Aortopulmonary Septal Defect. StatPearls (StatPearls Publishing, 2020).
- Persistent Truncus Arteriosus – Pediatrics – MSD Manual Professional Edition. https://www.msdmanuals.com/en-jp/professional/pediatrics/congenital-cardiovascular-anomalies/persistent-truncus-arteriosus.
- Total anomalous pulmonary venous return | Radiology Reference Article | Radiopaedia.org. https://radiopaedia.org/articles/total-anomalous-pulmonary-venous-return?lang=gb.
- Bass, D. & Bhimji, S. S. Aneurysm, Sinus Of Valsalva. in StatPearls (StatPearls Publishing, 2018).