Introduction
Transposition means ‘in each other’s place’.
The Great Arteries are:
- The pulmonary artery – which normally carries blue (deoxygenated) blood from the right ventricle to the lungs.
- The aorta – which normally carries red (oxygenated) blood from the left ventricle to the body.
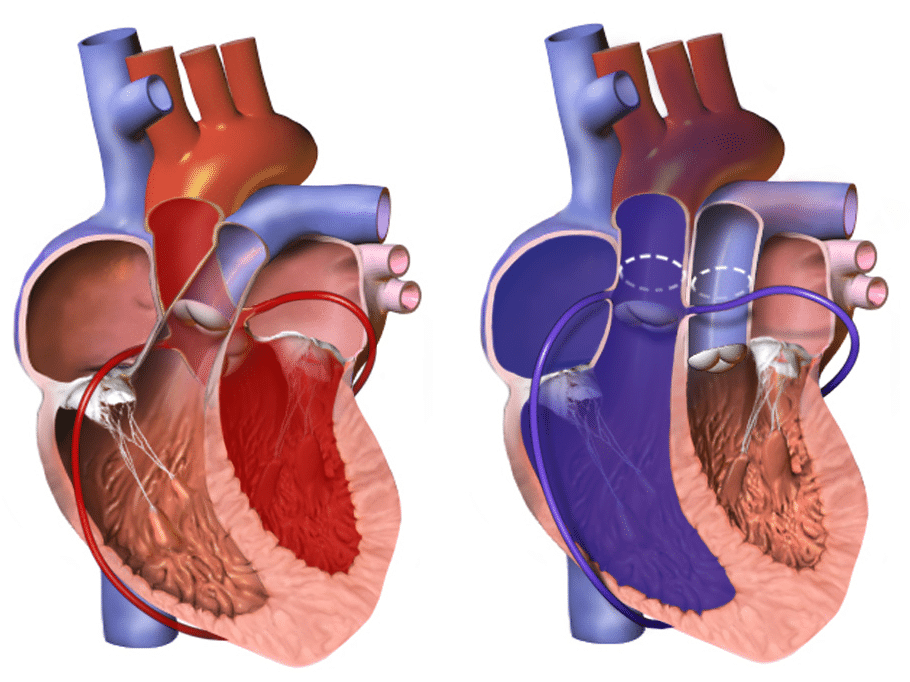
The hallmark of transposition of the great arteries is “ventriculoarterial discordance”, in which the aorta arises from the morphologic right ventricle and the pulmonary artery arises from the morphologic left ventricle
Although transposition of the great arteries was first described over 2 centuries ago, no treatment was available until the middle of the 20th century
The anatomical configuration of this anomaly establishes a potentially fatal parallel circulation that results in deep hypoxaemia from lack of mixing, with resulting lactic acidosis and demise. Prompt, adequate preoperative intervention and stabilization, followed by surgical repair and expert postoperative management, favour an excellent outcome.
Classification
In approximately 60% of the patients, the aorta is anterior and to the right of the pulmonary artery (dextro-transposition of the great arteries [d-TGA]).
However in a subset of patients, the aorta may be anterior and to the left of the pulmonary artery (levo-transposition of the great arteries [l-TGA])
In approximately one third of patients with transposition of the great arteries, the coronary artery anatomy is abnormal.
Epidemiology
- TGA is the most common cause of cyanosis in the new born
- TGA occurs in 5 –7 % of all CHD, that is approximately 20 – 30 incidences per 100,000 live births
- Incidence is higher in male infants, approximately 60-70%
Pathophysiology
In dextro-TGA the pulmonary and systemic circulation run in parallel, causing oxygenated blood to recirculate only in the pulmonary circulation and deoxygenated systemic blood to bypass the lungs. This results in cyanosis unless there is mixing of oxygenated blood and deoxygenated blood.
3 common anatomic sites for mixing of oxygenated and deoxygenated blood in transposition of the great arteries to allow life to be sustained:
- Patent foramen ovale or atrial septal defect
- Ventricular septal defect
- Patent ductus arteriosus
In levo-TGA [also called as CC-TGA] the ventricles have switched places as opposed to the arteries and thus this is acyanotic as deoxygenated blood can return from the systemic circulation and enter the pulmonary circulation to be oxygenated before entering the systemic circulation again. Nevertheless, the right ventricle and tricuspid valve is not accustomed to the higher pressures of the left side of the heart and thus, there is hypertrophy over time, which can result in tricuspid regurgitation and heart failure.
Aetiology
The exact embryological mechanisms that result in TGA is unknown; however, there are currently two theories that try to explain the phenomenon.
- Goor and Edwards suggest that the aorta does not rotate normally towards the left ventricle embryologically and imply that TGA is an extreme form of dextroposition of the aorta
- De la Cruz theorises that there is no rotation of the aorto-pulmonary septum at the infundibular level. This causes the fourth aortic arch, which will later become the aorta, to interact with the anterior conus on the right ventricle
Maternal Risk Factors
- Age is over 40 years old
- Maternal diabetes
- Rubella
- Poor nutrition
- Alcohol consumption
Clinical Features
From history
- Cyanosis appears in first 24 hours [if no mixing at the atrial level]
- Mild cyanosis (particularly when crying) might be evident. Signs of congestive heart failure (tachypnoea, tachycardia, diaphoresis, and failure to gain weight) may become evident over the first 3-6 weeks as pulmonary blood flow increases if large VSD present
From examination
- Prominent right ventricular heave
- Single second heart sound, loud A2
- Systolic murmur potentially if VSD present
- No signs of respiratory distress
Differential Diagnosis
Important differentials with salient features to help to differentiate
| Other congenital heart disease that causes cyanosis include: | How to differentiate it from TGA: |
| Tetralogy of Fallot (TOF) | CXR: boot-shaped heart
Echocardiogram: pulmonary cyanosis, overriding aorta, VSD and right ventricular hypertrophy |
| Tricuspid Atresia | ECG: left axis deviation |
Investigations
Pulse oximetry shows cyanosis and there can be discrepancy between upper and lower limbs. Capillary blood gas may show metabolic acidosis with decreased PaO2. As there is a lack of oxygen going to distal organs, cells respire anaerobically producing lactate.
Imaging or invasive tests:
- Echocardiogram: definitive for diagnosis. It shows the abnormal position of the aorta and pulmonary arteries
- CXR: “egg on a string” due to potentially narrowed upper mediastinum; cardiomegaly and increased pulmonary vascular markings
Management
Initial management:
- Emergency prostaglandin E1 infusion to keep the ductus arteriosus patent as a temporary solution that allows mixing of blood
- Correct metabolic acidosis
- Emergency atrial balloon septostomy to allow for mixing
Definitive and Long‐term management:
- Surgical correction, commonly arterial switch operation [ASO] is usually performed before the age of 4 weeks.
- Long term follow up and counselling in the future if female patients wish to get pregnant
Complications and Prognosis
Prognosis depends on the complexity of the TGA and how it was surgically repaired. Post-surgical correction, survival is approximately greater than 90% at 20 years.
Several long-term consequences are recognised. These include:
- Neopulmonary stenosis
- Neoaortic regurgitation
- Neoaortic root dilatation
- Coronary artery disease
- Anywhere between 2% to 8% of patients may require intervention, including balloon angioplasty, transcatheter stenting or surgical patch arterioplasty.
- Obstructed coronary arteries are present in 5% to 7% of survivors and remain the most common cause of morbidity and mortality after ASO. The incidence of myocardial ischaemia, infarction and death is highest in the first three months after ASO.
- The incidence of sudden cardiac death in repaired TGA patients is reported to be between 0.3% to 0.8%. This is thought to be related to primary arrhythmia.
- There is a high incidence frequency of neurodevelopmental (ND) abnormalities in these patients. All TGA patients should have ND evaluation ideally in early childhood.
- Low gestational age and a high pre-operative lactate are the most important predictors of poor developmental outcome
References
| [1] | M. Unolt, C. Putotto, L. Silvestri, D. Marino, A. Scarabotti, V. Massaccesi, A. Caiaro, P. Versacci and B. Marino, “Transposition of great arteries: new insights into the,” Frontiers in Paediatrics, vol. 1, pp. 1-7, 2013. |
| [2] | [Online]. Available: emedicine.medscape.com. |
| [3] | [Online]. Available: http://bestpractice.bmj.com. |
| [4] | [Online]. Available: http://www.chfed.org.uk. |
| [5] | [online]- htttp://www.radiopedia.org |