Introduction
Hypoplastic left heart syndrome (HLHS) as the term suggests, refers to the abnormal development of the left-sided cardiac structures, resulting in obstruction to blood flow from the left ventricular outflow tract.
Initially termed hypoplasia of the aortic tract complex by Lev in 1952. The series of Lev was followed by the study of Noonan and Nadas, who in 1958 first used the term hypoplastic left heart syndrome collectively to describe their series of specimens with multiple malformations involving left-sided structures of the heart.
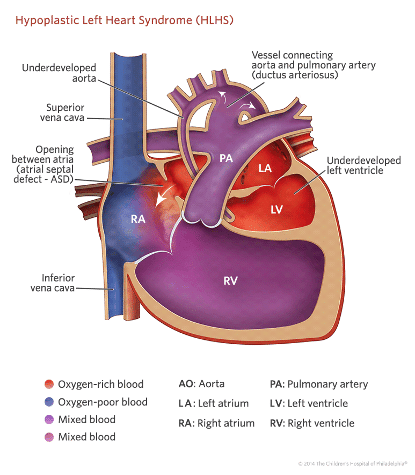
Figure 1: Hypoplastic left heart (1- hypoplastic aortic arch, 2-large PDA, 3 – hypoplastic left ventricle)
Epidemiology
Hypoplastic left heart syndrome occurs in 1 in 5000 live births [1]. It is more common in males. It accounts for 1 to 3.8 % of all congenital cardiac malformations. The recurrence risk in siblings is 0.5% [2].
Risk factors
Many previous studies have hypothesized and implicated environmental factors, seasonal variations and in utero maternal infections such as rubella, herpes virus, coxsackie virus, cytomegalovirus in association with the occurrence of HLHS. But no one factor has been strongly linked to the occurrence of hypoplastic left heart syndrome as yet.
Pathophysiology
By definition the left side of the heart is unable to support a systemic circulation.
The survival is dependent on a two key factors:
- a) Patent ductus arteriosus to ensure adequate systemic circulation and
- b) A non-restrictive atrial septal defect to ensure adequate mixing of oxygenated and deoxygenated blood.
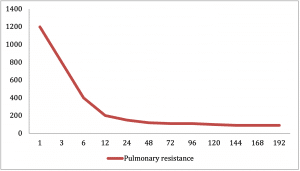
The right ventricle has to support both the systemic and pulmonary circulations. The relative distribution of cardiac output to the systemic and pulmonary circulations is dependent on the resistance in these parallel circuits. This factor plays a vital role in the presentation of this condition.
At birth the patent ductus arteriosus is usually unrestrictive and the pulmonary vascular resistance is relatively high. As a result there is no restriction of flow and so there is adequate systemic perfusion across the duct into the descending aorta. During this period neonates may be relatively asymptomatic. However, the two normal physiological events occurring over the initially few days of life, namely closure of PDA and reduction in pulmonary vascular resistance lead to a decrease in systemic perfusion and increase in pulmonary blood flow. This leads to the presentation of these neonates in cardiogenic shock.
All the structures of the left side of the heart are inter-related in their function and development and no single component can be considered in isolation in hypoplastic left heart syndrome. Thus the mitral valve, left ventricular cavity, left ventricular outflow tract, aortic valve, ascending aorta and aortic arch are all affected by the condition and frequently more than one or all entities are underdeveloped. It is also now postulated that many cases of HLHS are dynamic and progressive during gestation, resulting from altered LV inflow or outflow. This is a very promising theory that could be utilized in the field of prenatal cardiac intervention, and help to significantly improve outcomes in these cases.
Spectrum of HLHS: Due to the above mentioned dynamic evolution of the condition during fetal life, HLHS exists as a spectrum of defects as opposed to a single uniform presentation.
- Aortic atresia with mitral atresia (most extreme)
- Aortic atresia with patent mitral valve
- Aortic stenosis with patent mitral valve
Common cardiac associations:
- Coronary cameral fistulas (abnormal communication between coronaries and cardiac chamber) seen in patients with aortic atresia and patent mitral valve
- Persistent left SVC (15%)
- Anomalous pulmonary venous drainage (5-10%)
Other associations:
- Identified heritable syndromes associated with HLHS include: Kabuki syndrome, Noonan syndrome, Smith-Lemli-Opitz syndrome, Holt-Oram syndrome, CHARGE syndrome and PAGOD syndrome.
- Common trisomies and monosomies associated: Turners syndrome, trisomy 13, trisomy 18 and trisomy 21.
- Frequently associated CNS malformations: Agenesis of corpus callosum, holoprosencephaly and microcephaly.
Clinical features
Newborn infants generally are born at full term, and initially appear healthy. With closure of the arterial duct, the systemic perfusion becomes decreased, resulting in hypoxemia, acidosis, and shock.
Patients with a restrictive patent foramen ovale or intact atrial septum have pulmonary venous congestion and are cyanosed and tachypnoeic from birth. Restriction to flow of blood from the left to right atrium can result in severe left atrial hypertension, and decreased blood flow into the right atrium and the dominant right ventricle, resulting in decreased flows into both the systemic and pulmonary circulations and these babies present with cardiogenic shock.
Clinical signs:
- Tachycardia, dyspnea and evidence of pulmonary oedema
- Weak peripheral pulses, and vasoconstricted extremities
- Loud single S2 (due to aortic atresia)
- Hepatomegaly (secondary to congestive heart failure)
- ECG: shows RVH (and occasionally right axis deviation)
- CXR: shows pulmonary venous congestion or pulmonary edema. Moderately enlarged cardiac shadow.
- ECHO: Diminutive left ventricle, Dilated and enlarged right ventricle, Aortic hypoplasia, Color flow Doppler shows retrograde blood flow in aorta
Differential diagnosis
- Aortic stenosis
- Unbalanced AVSD
- Co-arctation of aorta
- Interrupted aortic arch
- TAPVD
Management
Initial stabilisation
Main aim of initial management is to secure patency of the duct:
- Prostaglandin E2 infusion
- Diuretics and inotropic support – in case of congestive cardiac failure
- Intubation and ventilation – occasionally required for hemodynamic stabilization
- Balloon atrial septostomy – may be required in cases of restrictive IAS
Definitive stabilisation
The central theme for the definitive management is conversion of the single ventricle into a systemic ventricle and establishing an obstructed pulmonary blood flow by bypassing the heart.
The definitive repair of HLHS is a 3-staged repair:
- Stage 1: Norwood procedure
- Stage 2: Glenn procedure (Bi-directional Glenn or Hemi-Fontan)
- Stage 3: Fontan Procedure
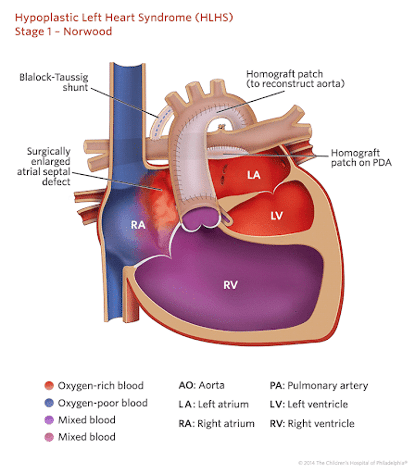
Stage 1: Norwood procedure
The main aims of the Norwood procedure are: securing an unobstructed systemic circulation and securing a balanced pulmonary blood flow.
The 4 components of the Norwood procedure are as follows :
- Atrial septectomy to establish unobstructed pulmonary venous return
- Reconstruction of the aortic arch using the main pulmonary artery to establish a systemic circulation
- Placement of a modified BT shunt /RV-PA conduit (Sano shunt) to re-establish pulmonary blood flow
- Creating a connection between smaller ascending aorta and pulmonary root to establish coronary blood supply (DKS – Dammus-Kaye Stansel)
One of the recent modifications to this procedure is the replacement of a modified BT shunt (connecting the right subclavian artery to the pulmonary artery) with a non-valved conduit connecting the right ventricle to the pulmonary artery referred to as the Sano shunt. The primary reason behind this modification is a phenomenon referred to as coronary steal that is seen in association with the BT shunt. As the pulmonary vascular resistance is lower than the systemic vascular resistance, blood flows from the aortic root to the pulmonary root during diastole. This results in decreased diastolic blood pressure in the aortic root and consequently a decreased coronary arterial perfusion and resultant myocardial ischaemia.
The Norwood procedure is the best balance that can be achieved given the substrate, but it remains one of the highest risk procedures in pediatric cardiac surgery. The surgical mortality rate during the Norwood procedure is between 7% to 19% [1].
Some centres also use a Hybrid procedure as a replacement to the conventional Norwood procedure, which is later followed on by a Fontan- type procedure. It is an innovative procedure performed by the interventional cardiologists and cardiac surgeons.
This procedure involves:
- Bilateral pulmonary artery banding – which helps to achieve adequate pulmonary blood flow without causing pulmonary hypertension or signs of heart failure.
- Placing a stent in the PDA to ensure its continued patency – which helps to achieve balanced systemic circulation.
The main advantage of such a procedure is that it helps to achieve a stable systemic and pulmonary circulation without having to undergo open heart surgery using cardio-pulmonary bypass during the neonatal period that carries a significantly high risk as previously explained. But the long term outcome of this procedure remains unclear and hence has not been widely adapted into practice.
Interstage management:
Recovery from the critical post-op period, marks the transition to a period of vigilance and monitoring. The time between stage 1 and stage 2 surgeries carries a significant mortality rate of 12% [1].
These incidences were seen to be more commonly associated with a modified BT shunt as opposed to a Sano shunt, which has been explained above. The other common factors associated with mortality are: residual coarctation, significant tricuspid regurgitation, shunt obstruction, volume overload of residual ventricle and left pulmonary artery stenosis.
Medical management post- Norwood procedure remains variable among a lot of institutions. They commonly include:
- Small dose diuretics: to reduce pulmonary over circulation and manage signs of heart failure
- Angiotensin converting enzyme inhibitors (Captopril): for afterload reduction
- Anti-platelet agents such as Aspirin/Clopidogrel: to prevent shunt thrombosis
- Digoxin: given occasionally to improve cardiac output.
The other measures that are commonly implemented are nutritional support, home monitoring of pulse oximetry, education of parents to seek advice if O2 saturation falls below 75%, this helps in early pick up of respiratory illnesses or insidious development of shunt thrombosis.
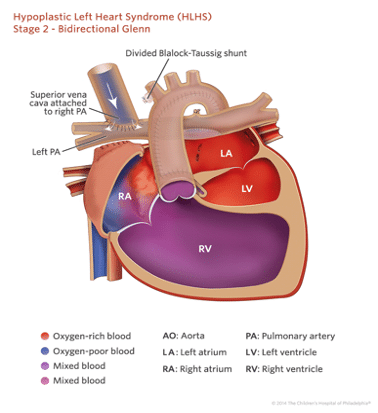
Stage 2: Glenn Procedure (Bi-directional Glenn/Hemi-Fontan)
The second stage procedure is done around 4-6 months, by which time children have doubled their birth weight approximately and are beginning to outgrow their previous shunt. Another very important change at this point is that the pulmonary vascular resistance has sufficiently fallen down to adult levels, and a high pressure circulation is not required any more for pulmonary blood supply.
This procedure can be preceded by a cardiac MRI or cardiac catheter to assess suitability to progress to stage 2.
This procedure involves anastomosis of the superior vena cava to the ipsilateral pulmonary artery and takedown of previously placed shunts to provide pulmonary blood flow. The patients are far more robust after the completion of the Glenn procedure, the post-Glenn circulation is characterized by a passive source of pulmonary blood flow, volume unloaded single systemic ventricle and persistent cyanosis due to flow of IVC return to RV. The post-op oxygen levels in these children are in the low 80’s.
Following this stage, the mortality rate is low (5%) and most children are initially fine with this level of systemic oxygenation [1]. As the children grow and their activity levels increase, the degree of cyanosis increases. The stage 3 procedure or the Fontan completion should then be considered.
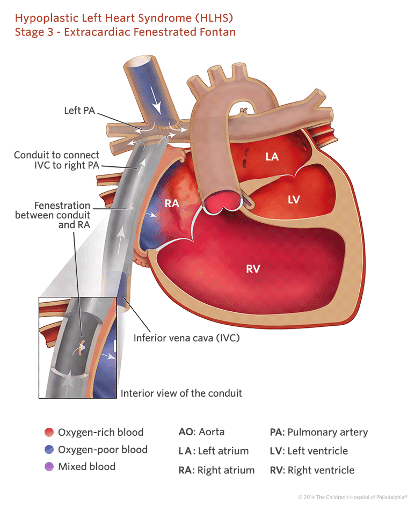
Stage 3: Fontan Procedure
This is the last anticipated operation in HLHS pathway. It is performed between 18 months to 4 years. This procedure directs inferior vena cava return to the pulmonary vasculature, so that all the systemic venous return runs passively to the lungs, effectively bypassing the right ventricle. This creates a system with a single ventricle pumping blood into separate, systemic and pulmonary circulations aligned in series, thereby relieving cyanosis.
The Fontan procedure was initially conceived by Francis Fontan about 30 years ago for other less complex forms of uni-ventricular heart disease. But it was then adapted for the hypoplastic left heart syndrome. It has undergone numerous technical modifications over the years and the two modern versions are:
- The lateral tunnel Fontan: achieved by an intra-atrial baffle to direct IVC return via a lateral tunnel into the pulmonary arteries.
- The extra-cardiac Fontan: achieved by connecting a large bore Gore-tex tube from IVC to underside of the pulmonary arteries.
The extra-cardiac approach has been shown to be superior to the lateral tunnel approach, as it is associated with lesser incidences of atrial arrhythmias.
The mortality rate for this procedure is about 3-4% and closely comparable to that of the Glenn procedure [3]. Outcomes for this procedure have been improved by the creation of fenestration between the conduit and the right atrium, this acts as a ‘pop-off valve’ that helps relieve some pressure in the Fontan circulation when the pulmonary vascular resistance is high. Since this allows for some blood to by-pass the pulmonary circulation and return directly to systemic circulation, a small degree of desaturation and cyanosis is expected. Patients with fenestrated Fontan circulation will have saturation in the low 80’s initially, which will gradually rise to the high 80’s. This modification to the Fontan circuit has been adapted by most centres.
The commonly mentioned long-term complications after the Fontan procedure are:
- Desaturation (in patients without an intentional fenerstration): this is most commonly the result of development of veno-venous collaterals (arising from the systemic venous vessels and draining to the pulmonary venous vessels).
- Cyanosis: which could be the result of Fontan circuit obstruction or development of pulmonary arterio-venous malformations.
- Arrhythmias: By 10 years post Fontan procedure approximately 20% of the patients have supraventricular arrhythmias.
- Heart Failure
Neonatal transplantation
Heart transplantation can be considered as an alternative to the staged repair approach. The technical aspects of the transplantation surgery are fairly standard but there is a severe shortage of donor hearts of a suitable size. It has previously been observed that roughly 25% of the infant population sadly, die while awaiting a donor heart [4]. Patients with history of HLHS experience greater morbidity and mortality when undergoing transplantation. Because of all these factors this option has never been pursued as a viable treatment option.
Long term outcomes
The above mentioned medical and surgical interventions have helped to change HLHS from a condition with a fatal prognosis to one that now has an estimated three to six year survival rate of 60-70%, for infants undergoing stage 1 repair. Infants who survive the initial 12 months, have a long term survival rate of approximately 90% (to the age of 18 years) [5]. It is also important to note that patients with HLHS who survive the staged palliation are at a risk of neurodevelopmental impairment.
The other aspect to be considered here is the ongoing ethical debate over the role of investment in a series of complex cardiac procedures to achieve what is deemed to be a palliative circulation. Previous studies have attempted to assess the quality of life of these children and most markers show that these children underperform in comparison with other healthy peers. Nevertheless most children who reach school age seem to have a normal intelligence quotient.
Key learning points
- Fetuses antenatally diagnosed with HLHS should be delivered and managed at tertiary care centres with expertise in medical and surgical treatment of HLHS.
- Neonates may present completely asymptomatic at birth, but develop hypoxaemia, acidosis and shock with the closure of the ductus arteriosus.
- Initial management is focused on ensuring adequate mixing of oxygenated and de-oxygenated blood (non-restrictive inter-atrial septum), ensuring adequate systemic perfusion (maintaining ductal patency)
- Surgical management consists of a 3 staged repair namely: Norwood procedure (neonatal age) -> Bi-directional Glen procedure (4-6 months) -> Fontan completion (18 months to 4 yrs)
- Stage 1 or Norwood procedure carries the highest mortality rate of up to 15% and continues to be one of the cardiac surgeries with highest risk.
- For children who survive the initial 12 months, long term survival rates are 90%.
- Children born with HLHS are at a high risk of developmental delay and should undergo periodic developmental screening assessments.
References
| (1) | Park’s Paediatric cardiology for practitioners: Cyanotic congenital heart defects |
| (2) | Moss and Adams’ Heart disease in Infants, Children and Adolescents (Volume 2): Hypoplastic left heart syndrome |
| (3) | Hirsch JC, Goldberg C, et al. Fontan operation in the current era: a 15-year single institution experience Ann Surg 2008; 248: 402–10. |
| (4) | Chrisant MR, Naftel DC et al. Pediatric heart transplant study group fate of infants with hypoplastic left heart syndrome listed for cardiac transplantation: a multicenter study.J Heart Lung Transplant 2005; 24: 576–82. |
| (5) | Siffel C, Riehle-Colarusso T et al. Survival of Children with Hypoplastic Left Heart Syndrome. Pediatrics 2015 Oct; 136(4): e864-70 |
Acknowledgments
All the images have been sourced from Children’s Hospital of Philadelphia Cardiac Centre website and Radiopaedia, and being reproduced with their permission.